Bayesian Diallel Analysis Reveals Mx1-Dependent and Mx1-Independent Effects on Response to Influenza A Virus in Mice
- PMID: 29187420
- PMCID: PMC5919740
- DOI: 10.1534/g3.117.300438
Bayesian Diallel Analysis Reveals Mx1-Dependent and Mx1-Independent Effects on Response to Influenza A Virus in Mice
Abstract
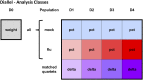
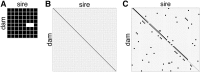
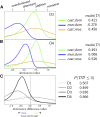
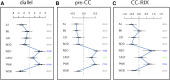
Influenza A virus (IAV) is a respiratory pathogen that causes substantial morbidity and mortality during both seasonal and pandemic outbreaks. Infection outcomes in unexposed populations are affected by host genetics, but the host genetic architecture is not well understood. Here, we obtain a broad view of how heritable factors affect a mouse model of response to IAV infection using an 8 × 8 diallel of the eight inbred founder strains of the Collaborative Cross (CC). Expanding on a prior statistical framework for modeling treatment response in diallels, we explore how a range of heritable effects modify acute host response to IAV through 4 d postinfection. Heritable effects in aggregate explained ∼57% of the variance in IAV-induced weight loss. Much of this was attributable to a pattern of additive effects that became more prominent through day 4 postinfection and was consistent with previous reports of antiinfluenza myxovirus resistance 1 (Mx1) polymorphisms segregating between these strains; these additive effects largely recapitulated haplotype effects observed at the Mx1 locus in a previous study of the incipient CC, and are also replicated here in a CC recombinant intercross population. Genetic dominance of protective Mx1 haplotypes was observed to differ by subspecies of origin: relative to the domesticus null Mx1 allele, musculus acts dominantly whereas castaneus acts additively. After controlling for Mx1, heritable effects, though less distinct, accounted for ∼34% of the phenotypic variance. Implications for future mapping studies are discussed.
Keywords: Bayesian mixed model; MPP; causal effect; multiparental populations; multiple imputation; treatment response.
Copyright © 2018 Maurizio et al.
Figures



Similar articles
-
Protection from Severe Influenza Virus Infections in Mice Carrying the Mx1 Influenza Virus Resistance Gene Strongly Depends on Genetic Background.J Virol. 2015 Oct;89(19):9998-10009. doi: 10.1128/JVI.01305-15. Epub 2015 Jul 22. J Virol. 2015. PMID: 26202236 Free PMC article.
-
Influenza Virus Susceptibility of Wild-Derived CAST/EiJ Mice Results from Two Amino Acid Changes in the MX1 Restriction Factor.J Virol. 2016 Nov 14;90(23):10682-10692. doi: 10.1128/JVI.01213-16. Print 2016 Dec 1. J Virol. 2016. PMID: 27654285 Free PMC article.
-
Differential lung gene expression changes in C57BL/6 and DBA/2 mice carrying an identical functional Mx1 gene reveals crucial differences in the host response.BMC Genom Data. 2024 Feb 15;25(1):19. doi: 10.1186/s12863-024-01203-3. BMC Genom Data. 2024. PMID: 38360537 Free PMC article.
-
Mx genes: host determinants controlling influenza virus infection and trans-species transmission.Hum Genet. 2020 Jun;139(6-7):695-705. doi: 10.1007/s00439-019-02092-8. Epub 2019 Nov 26. Hum Genet. 2020. PMID: 31773252 Free PMC article. Review.
-
Disease resistance in farm animals.Experientia. 1991 Sep 15;47(9):923-34. doi: 10.1007/BF01929883. Experientia. 1991. PMID: 1915776 Review.
Cited by
-
Mouse Models as Resources for Studying Infectious Diseases.Clin Ther. 2019 Oct;41(10):1912-1922. doi: 10.1016/j.clinthera.2019.08.010. Epub 2019 Sep 18. Clin Ther. 2019. PMID: 31540729 Free PMC article. Review.
-
Characterization of Collaborative Cross mouse founder strain CAST/EiJ as a novel model for lethal COVID-19.Sci Rep. 2024 Oct 24;14(1):25147. doi: 10.1038/s41598-024-77087-1. Sci Rep. 2024. PMID: 39448712 Free PMC article.
-
The Collaborative Cross: A Systems Genetics Resource for Studying Host-Pathogen Interactions.Cell Host Microbe. 2019 Apr 10;25(4):484-498. doi: 10.1016/j.chom.2019.03.009. Cell Host Microbe. 2019. PMID: 30974083 Free PMC article. Review.
-
Sarbecovirus disease susceptibility is conserved across viral and host models.Virus Res. 2024 Aug;346:199399. doi: 10.1016/j.virusres.2024.199399. Epub 2024 Jun 14. Virus Res. 2024. PMID: 38823688 Free PMC article.
-
A Diallel of the Mouse Collaborative Cross Founders Reveals Strong Strain-Specific Maternal Effects on Litter Size.G3 (Bethesda). 2019 May 7;9(5):1613-1622. doi: 10.1534/g3.118.200847. G3 (Bethesda). 2019. PMID: 30877080 Free PMC article.
References
-
- Alberts R., Srivastava B., Wu H., Viegas N., Geffers R., et al. , 2010. Gene expression changes in the host response between resistant and susceptible inbred mouse strains after influenza A infection. Microbes Infect. 12: 309–318. - PubMed
-
- Bates D., Mächler M., Bolker B., Walker S., 2015. Fitting linear mixed-effects models using lme4. J. Stat. Softw. 67: 1–48.
-
- Boivin G. A., Pothlichet J., Skamene E., Brown E. G., Loredo-Osti J. C., et al. , 2012. Mapping of clinical and expression quantitative trait loci in a sex-dependent effect of host susceptibility to mouse-adapted influenza H3N2/HK/1/68. J. Immunol. 188: 3949–3960. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
