Novel Insect-Specific Eilat Virus-Based Chimeric Vaccine Candidates Provide Durable, Mono- and Multivalent, Single-Dose Protection against Lethal Alphavirus Challenge
- PMID: 29187545
- PMCID: PMC5790933
- DOI: 10.1128/JVI.01274-17
Novel Insect-Specific Eilat Virus-Based Chimeric Vaccine Candidates Provide Durable, Mono- and Multivalent, Single-Dose Protection against Lethal Alphavirus Challenge
Abstract
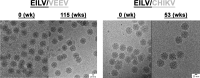
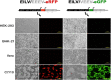
Most alphaviruses are mosquito borne and exhibit a broad host range, infecting many different vertebrates, including birds, rodents, equids, humans, and nonhuman primates. Recently, a host-restricted, mosquito-borne alphavirus, Eilat virus (EILV), was described with an inability to infect vertebrate cells based on defective attachment and/or entry, as well as a lack of genomic RNA replication. We investigated the utilization of EILV recombinant technology as a vaccine platform against eastern (EEEV) and Venezuelan equine encephalitis viruses (VEEV), two important pathogens of humans and domesticated animals. EILV chimeras containing structural proteins of EEEV or VEEV were engineered and successfully rescued in Aedes albopictus cells. Cryo-electron microscopy reconstructions at 8 and 11 Å of EILV/VEEV and EILV/EEEV, respectively, showed virion and glycoprotein spike structures similar to those of VEEV-TC83 and other alphaviruses. The chimeras were unable to replicate in vertebrate cell lines or in brains of newborn mice when injected intracranially. Histopathologic examinations of the brain tissues showed no evidence of pathological lesions and were indistinguishable from those of mock-infected animals. A single-dose immunization of either monovalent or multivalent EILV chimera(s) generated neutralizing antibody responses and protected animals against lethal challenge 70 days later. Lastly, a single dose of monovalent EILV chimeras generated protective responses as early as day 1 postvaccination and partial or complete protection by day 6. These data demonstrate the safety, immunogenicity, and efficacy of novel insect-specific EILV-based chimeras as potential EEEV and VEEV vaccines.IMPORTANCE Mostly in the last decade, insect-specific viruses have been discovered in several arbovirus families. However, most of these viruses are not well studied and largely have been ignored. We explored the use of the mosquito-specific alphavirus EILV as an alphavirus vaccine platform in well-established disease models for eastern (EEE) and Venezuelan equine encephalitis (VEE). EILV-based chimeras replicated to high titers in a mosquito cell line yet retained their host range restriction in vertebrates both in vitro and in vivo In addition, the chimeras generated immune responses that were higher than those of other human and/or equine vaccines. These findings indicate the feasibility of producing a safe, efficacious, mono- or multivalent vaccine against the encephalitic alphaviruses VEEV and EEEV. Lastly, these data demonstrate how host-restricted, insect-specific viruses can be engineered to develop vaccines against related pathogenic arboviruses that cause severe disease in humans and domesticated animals.
Keywords: Eilat virus; alphavirus; emerging infectious diseases; vaccines.
Copyright © 2018 American Society for Microbiology.
Figures







References
-
- Griffin DE. 2007. Alphaviruses, p 1023–1068. In Fields BN, Knipe DM, Howley PM, Fields virology, 6th ed Wolters Kluwer Health/Lippincott Williams & Wilkins, Philadelphia, PA.
-
- Nasar F, Palacios G, Gorchakov RV, Guzman H, Da Rosa AP, Savji N, Popov VL, Sherman MB, Lipkin WI, Tesh RB, Weaver SC. 2012. Eilat virus, a unique alphavirus with host range restricted to insects by RNA replication. Proc Natl Acad Sci U S A 109:14622–14627. doi: 10.1073/pnas.1204787109. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

