Pathogenesis, Humoral Immune Responses, and Transmission between Cohoused Animals in a Ferret Model of Human Respiratory Syncytial Virus Infection
- PMID: 29187546
- PMCID: PMC5790937
- DOI: 10.1128/JVI.01322-17
Pathogenesis, Humoral Immune Responses, and Transmission between Cohoused Animals in a Ferret Model of Human Respiratory Syncytial Virus Infection
Abstract
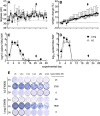
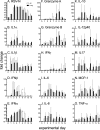
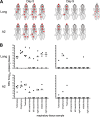
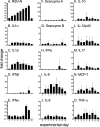
Small-animal models have been used to obtain many insights regarding the pathogenesis and immune responses induced following infection with human respiratory syncytial virus (hRSV). Among those described to date, infections in cotton rats, mice, guinea pigs, chinchillas, and Syrian hamsters with hRSV strains Long and/or A2 have been well characterized, although clinical isolates have also been examined. Ferrets are also susceptible to hRSV infection, but the pathogenesis and immune responses elicited following infection have not been well characterized. Here, we describe the infection of adult ferrets with hRSV Long or A2 via the intranasal route and characterized virus replication, as well as cytokine induction, in the upper and lower airways. Virus replication and cytokine induction during the acute phase of infection (days 0 to 15 postinfection) were similar between the two strains, and both elicited high levels of F glycoprotein-specific binding and neutralizing antibodies following virus clearance (days 16 to 22 postinfection). Importantly, we demonstrate transmission from experimentally infected donor ferrets to cohoused naive recipients and have characterized virus replication and cytokine induction in the upper airways of infected contact animals. Together, these studies provide a direct comparison of the pathogenesis of hRSV Long and A2 in ferrets and highlight the potential of this animal model to study serological responses and examine interventions that limit transmission of hRSV.IMPORTANCE Ferrets have been widely used to study pathogenesis, immunity, and transmission following human influenza virus infections; however, far less is known regarding the utility of the ferret model to study hRSV infections. Following intranasal infection of adult ferrets with the well-characterized Long or A2 strain of hRSV, we report virus replication and cytokine induction in the upper and lower airways, as well as the development of virus-specific humoral responses. Importantly, we demonstrate transmission of hRSV from experimentally infected donor ferrets to cohoused naive recipients. Together, these findings significantly enhance our understanding of the utility of the ferret as a small-animal model to investigate aspects of hRSV pathogenesis and immunity.
Keywords: animal models; ferret; respiratory syncytial virus; respiratory viruses; transmission.
Copyright © 2018 American Society for Microbiology.
Figures



References
-
- Afonso CL, Amarasinghe GK, Banyai K, Bao Y, Basler CF, Bavari S, Bejerman N, Blasdell KR, Briand FX, Briese T, Bukreyev A, Calisher CH, Chandran K, Cheng J, Clawson AN, Collins PL, Dietzgen RG, Dolnik O, Domier LL, Durrwald R, Dye JM, Easton AJ, Ebihara H, Farkas SL, Freitas-Astua J, Formenty P, Fouchier RA, Fu Y, Ghedin E, Goodin MM, Hewson R, Horie M, Hyndman TH, Jiang D, Kitajima EW, Kobinger GP, Kondo H, Kurath G, Lamb RA, Lenardon S, Leroy EM, Li CX, Lin XD, Liu L, Longdon B, Marton S, Maisner A, Muhlberger E, Netesov SV, Nowotny N, et al. 2016. Taxonomy of the order Mononegavirales: update 2016. Arch Virol 161:2351–2360. doi: 10.1007/s00705-016-2880-1. - DOI - PMC - PubMed
-
- Nair H, Nokes DJ, Gessner BD, Dherani M, Madhi SA, Singleton RJ, O'Brien KL, Roca A, Wright PF, Bruce N, Chandran A, Theodoratou E, Sutanto A, Sedyaningsih ER, Ngama M, Munywoki PK, Kartasasmita C, Simoes EA, Rudan I, Weber MW, Campbell H. 2010. Global burden of acute lower respiratory infections due to respiratory syncytial virus in young children: a systematic review and meta-analysis. Lancet 375:1545–1555. doi: 10.1016/S0140-6736(10)60206-1. - DOI - PMC - PubMed
-
- Scheltema NM, Gentile A, Lucion F, Nokes DJ, Munywoki PK, Madhi SA, Groome MJ, Cohen C, Moyes J, Thorburn K, Thamthitiwat S, Oshitani H, Lupisan SP, Gordon A, Sanchez JF, O'Brien KL, Group PS, Gessner BD, Sutanto A, Mejias A, Ramilo O, Khuri-Bulos N, Halasa N, de-Paris F, Pires MR, Spaeder MC, Paes BA, Simoes EAF, Leung TF, da Costa Oliveira MT, de Freitas Lazaro Emediato CC, Bassat Q, Butt W, Chi H, Aamir UB, Ali A, Lucero MG, Fasce RA, Lopez O, Rath BA, Polack FP, Papenburg J, Roglic S, Ito H, Goka EA, Grobbee DE, Nair H, Bont LJ. 2017. Global respiratory syncytial virus-associated mortality in young children (RSV GOLD): a retrospective case series. Lancet Glob Health 5:e984–e991. doi: 10.1016/S2214-109X(17)30344-3. - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

