Development and Validation of a Real-Time PCR Assay for Rapid Detection of Candida auris from Surveillance Samples
- PMID: 29187562
- PMCID: PMC5786737
- DOI: 10.1128/JCM.01223-17
Development and Validation of a Real-Time PCR Assay for Rapid Detection of Candida auris from Surveillance Samples
Abstract
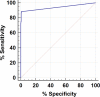
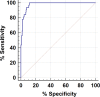
Candida auris is an emerging multidrug-resistant yeast causing invasive health care-associated infection with high mortality worldwide. Rapid identification of C. auris is of primary importance for the implementation of public health measures to control the spread of infection. To achieve these goals, we developed and validated a TaqMan-based real-time PCR assay targeting the internal transcribed spacer 2 (ITS2) region of the ribosomal gene. The assay was highly specific, reproducible, and sensitive, with the detection limit of 1 C. auris CFU/PCR. The performance of the C. auris real-time PCR assay was evaluated by using 623 surveillance samples, including 365 patient swabs and 258 environmental sponges. Real-time PCR yielded positive results from 49 swab and 58 sponge samples, with 89% and 100% clinical sensitivity with regard to their respective culture-positive results. The real-time PCR also detected C. auris DNA from 1% and 12% of swab and sponge samples with culture-negative results, indicating the presence of dead or culture-impaired C. auris The real-time PCR yielded results within 4 h of sample processing, compared to 4 to 14 days for culture, reducing turnaround time significantly. The new real-time PCR assay allows for accurate and rapid screening of C. auris and can increase effective control and prevention of this emerging multidrug-resistant fungal pathogen in health care facilities.
Keywords: Candida auris; TaqMan chemistry; assay validation; real-time PCR assay; surveillance samples.
Copyright © 2018 Leach et al.
Figures


References
-
- Lockhart SR, Etienne KA, Vallabhaneni S, Farooqi J, Chowdhary A, Govender NP, Colombo AL, Calvo B, Cuomo CA, Desjardins CA, Berkow EL, Castanheira M, Magobo RE, Jabeen K, Asghar RJ, Meis JF, Jackson B, Chiller T, Litvintseva AP. 2017. Simultaneous emergence of multidrug-resistant Candida auris on 3 continents confirmed by whole-genome sequencing and epidemiological analyses. Clin Infect Dis 64:134–140. doi:10.1093/cid/ciw691. - DOI - PMC - PubMed
-
- Vallabhaneni S, Kallen A, Tsay S, Chow N, Welsh R, Kerins J, Kemble SK, Pacilli M, Black SR, Landon E, Ridgway J, Palmore TN, Zelzany A, Adams EH, Quinn M, Chaturvedi S, Greenko J, Fernandez R, Southwick K, Furuya EY, Calfee DP, Hamula C, Patel G, Barrett P, Msd Lafaro P, Berkow EL, Moulton-Meissner H, Noble-Wang J, Fagan RP, Jackson BR, Lockhart SR, Litvintseva AP, Chiller TM. 2016. Investigation of the first seven reported cases of Candida auris, a globally emerging invasive, multidrug-resistant fungus—United States, May 2013–August 2016. MMWR Morb Mortal Wkly Rep 65:1234–1237. doi:10.15585/mmwr.mm6544e1. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

