Rescue of Pompe disease in mice by AAV-mediated liver delivery of secretable acid α-glucosidase
- PMID: 29187643
- PMCID: PMC5826611
- DOI: 10.1126/scitranslmed.aam6375
Rescue of Pompe disease in mice by AAV-mediated liver delivery of secretable acid α-glucosidase
Abstract
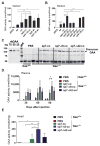
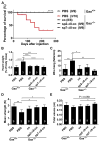
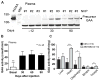
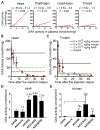
Glycogen storage disease type II or Pompe disease is a severe neuromuscular disorder caused by mutations in the lysosomal enzyme, acid α-glucosidase (GAA), which result in pathological accumulation of glycogen throughout the body. Enzyme replacement therapy is available for Pompe disease; however, it has limited efficacy, has high immunogenicity, and fails to correct pathological glycogen accumulation in nervous tissue and skeletal muscle. Using bioinformatics analysis and protein engineering, we developed transgenes encoding GAA that could be expressed and secreted by hepatocytes. Then, we used adeno-associated virus (AAV) vectors optimized for hepatic expression to deliver the GAA transgenes to Gaa knockout (Gaa-/-) mice, a model of Pompe disease. Therapeutic gene transfer to the liver rescued glycogen accumulation in muscle and the central nervous system, and ameliorated cardiac hypertrophy as well as muscle and respiratory dysfunction in the Gaa-/- mice; mouse survival was also increased. Secretable GAA showed improved therapeutic efficacy and lower immunogenicity compared to nonengineered GAA. Scale-up to nonhuman primates, and modeling of GAA expression in primary human hepatocytes using hepatotropic AAV vectors, demonstrated the therapeutic potential of AAV vector-mediated liver expression of secretable GAA for treating pathological glycogen accumulation in multiple tissues in Pompe disease.
Copyright © 2017 The Authors, some rights reserved; exclusive licensee American Association for the Advancement of Science. No claim to original U.S. Government Works.
Conflict of interest statement
Figures



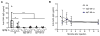
Comment in
-
Pompe disease: how to solve many problems with one solution.Ann Transl Med. 2018 Aug;6(15):313. doi: 10.21037/atm.2018.06.52. Ann Transl Med. 2018. PMID: 30211201 Free PMC article. No abstract available.
References
-
- van der Ploeg AT, Reuser AJJ. Pompe’s disease. Lancet. 2008;372:1342–1353. - PubMed
-
- Kishnani PS, Corzo D, Nicolino M, Byrne B, Mandel H, Hwu WL, Leslie N, Levine J, Spencer C, McDonald M, Li J, Dumontier J, Halberthal M, Chien YH, Hopkin R, Vijayaraghavan S, Gruskin D, Bartholomew D, van der Ploeg A, Clancy JP, Parini R, Morin G, Beck M, De la Gastine GS, Jokic M, Thurberg B, Richards S, Bali D, Davison M, Worden MA, Chen YT, Wraith JE. Recombinant human acid α-glucosidase: Major clinical benefits in infantile-onset Pompe disease. Neurology. 2007;68:99–109. - PubMed
-
- Schoser B, Stewart A, Kanters S, Hamed A, Jansen J, Chan K, Karamouzian M, Toscano A. Survival and long-term outcomes in late-onset Pompe disease following alglucosidase alfa treatment: A systematic review and meta-analysis. J Neurol. 2017;264:621–630. - PubMed
-
- van der Ploeg AT, Clemens PR, Corzo D, Escolar DM, Florence J, Groeneveld GJ, Herson S, Kishnani PS, Laforet P, Lake SL, Lange DJ, Leshner RT, Mayhew JE, Morgan C, Nozaki K, Park DJ, Pestronk A, Rosenbloom B, Skrinar A, van Capelle CI, van der Beek NA, Wasserstein M, Zivkovic SA. A randomized study of alglucosidase alfa in late-onset Pompe’s disease. N Engl J Med. 2010;362:1396–1406. - PubMed
-
- Kishnani PS, Beckemeyer AA. New therapeutic approaches for Pompe disease: Enzyme replacement therapy and beyond. Pediatr Endocrinol Rev. 2014;12(suppl 1):114–124. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

