Differences in the Thoracic Aorta by Region and Sex in a Murine Model of Marfan Syndrome
- PMID: 29187826
- PMCID: PMC5694786
- DOI: 10.3389/fphys.2017.00933
Differences in the Thoracic Aorta by Region and Sex in a Murine Model of Marfan Syndrome
Abstract
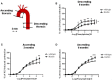
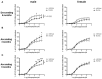
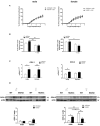
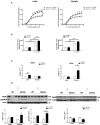
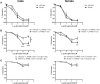
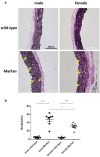
Marfan syndrome (MFS) is a hereditary disorder of the connective tissue that causes life-threatening aortic aneurysm, which initiates at the aortic root and can progress into the ascending portion. However, analysis of ascending aorta reactivity in animal models of MFS has remained elusive. Epidemiologic evidence suggests that although MFS is equally prevalent in men and women, men are at a higher risk of aortic complications than non-pregnant women. Nevertheless, there is no experimental evidence to support this hypothesis. The aim of this study was to explore whether there are regional and sex differences in the thoracic aorta function of mice heterozygous for the fibrillin 1 (Fbn1) allele encoding a missense mutation (Fbn1C1039G/+), the most common class of mutation in MFS. Ascending and descending thoracic aorta reactivity was evaluated by wire myography. Ascending aorta mRNA and protein levels, and elastic fiber integrity were assessed by qRT-PCR, Western blotting, and Verhoeff-Van Gieson histological staining, respectively. MFS differently altered reactivity in the ascending and descending thoracic aorta by either increasing or decreasing phenylephrine contractions, respectively. When mice were separated by sex, contractions to phenylephrine increased progressively from 3 to 6 months of age in MFS ascending aortas of males, whereas contractions in females were unchanged. Endothelium-dependent relaxation was unaltered in the MFS ascending aorta of either sex; an effect related to augmented endothelium-dependent hyperpolarization-type dilations. In MFS males, the non-selective cyclooxygenase (COX) inhibitor indomethacin prevented the MFS-induced enhancement of phenylephrine contractions linked to increased COX-2 expression. In MFS mice of both sexes, the non-selective nitric oxide synthase inhibitor L-NAME revealed negative feedback of nitric oxide on phenylephrine contractions, which was associated with upregulation of eNOS in females. Finally, MFS ascending aortas showed a greater number of elastic fiber breaks than the wild-types, and males exhibited more breaks than females. These results show regional and sex differences in Fbn1C1039G/+ mice thoracic aorta contractility and aortic media injuries. The presence of more pronounced aortic alterations in male mice provides experimental evidence to support that male MFS patients are at increased risk of suffering aortic complications.
Keywords: Marfan syndrome; aortic aneurysm; ascending thoracic aorta contraction; cyclooxygenase; elastin fragmentation; gender medicine; nitric oxide synthase; sex differences.
Figures
Similar articles
-
Fibrillin-1 Deficiency Perturbs Aortic Cholinergic Relaxation and Adrenergic Contraction in a Mouse Model of Early Onset Progressively Severe Marfan Syndrome.J Vasc Res. 2025;62(2):96-108. doi: 10.1159/000542481. Epub 2025 Jan 17. J Vasc Res. 2025. PMID: 39827863 Free PMC article.
-
Endothelial dysfunction and compromised eNOS/Akt signaling in the thoracic aorta during the progression of Marfan syndrome.Br J Pharmacol. 2007 Apr;150(8):1075-83. doi: 10.1038/sj.bjp.0707181. Epub 2007 Mar 5. Br J Pharmacol. 2007. PMID: 17339838 Free PMC article.
-
Genetic Manipulation of Caveolin-1 in a Transgenic Mouse Model of Aortic Root Aneurysm: Sex-Dependent Effects on Endothelial and Smooth Muscle Function.Int J Mol Sci. 2024 Nov 26;25(23):12702. doi: 10.3390/ijms252312702. Int J Mol Sci. 2024. PMID: 39684412 Free PMC article.
-
Cardiovascular characteristics in Marfan syndrome and their relation to the genotype.Verh K Acad Geneeskd Belg. 2009;71(6):335-71. Verh K Acad Geneeskd Belg. 2009. PMID: 20232788 Review.
-
Nitric oxide in the Marfan vasculature: Friend or foe?Nitric Oxide. 2021 Nov 1;116:27-34. doi: 10.1016/j.niox.2021.08.006. Epub 2021 Aug 31. Nitric Oxide. 2021. PMID: 34478846 Review.
Cited by
-
Sensitive asprosin detection in clinical samples reveals serum/saliva correlation and indicates cartilage as source for serum asprosin.Sci Rep. 2022 Jan 25;12(1):1340. doi: 10.1038/s41598-022-05060-x. Sci Rep. 2022. PMID: 35079041 Free PMC article.
-
Reactive Oxygen Species and Oxidative Stress in the Pathogenesis and Progression of Genetic Diseases of the Connective Tissue.Antioxidants (Basel). 2020 Oct 19;9(10):1013. doi: 10.3390/antiox9101013. Antioxidants (Basel). 2020. PMID: 33086603 Free PMC article. Review.
-
Oral Health-Related Quality of Life in People with Rare Hereditary Connective Tissue Disorders: Marfan Syndrome.Int J Environ Res Public Health. 2018 Oct 27;15(11):2382. doi: 10.3390/ijerph15112382. Int J Environ Res Public Health. 2018. PMID: 30373236 Free PMC article.
-
Fibrillin-1 Deficiency Perturbs Aortic Cholinergic Relaxation and Adrenergic Contraction in a Mouse Model of Early Onset Progressively Severe Marfan Syndrome.J Vasc Res. 2025;62(2):96-108. doi: 10.1159/000542481. Epub 2025 Jan 17. J Vasc Res. 2025. PMID: 39827863 Free PMC article.
-
PHACTR1 modulates vascular compliance but not endothelial function: a translational study.Cardiovasc Res. 2023 Mar 31;119(2):599-610. doi: 10.1093/cvr/cvac092. Cardiovasc Res. 2023. PMID: 35653516 Free PMC article.
References
-
- Brandes R. P., Schmitz-Winnenthal F. H., Félétou M., Gödecke A., Huang P. L., Vanhoutte P. M., et al. (2000). An endothelium-derived hyperpolarizing factor distinct from NO and prostacyclin is a major endothelium-dependent vasodilator in resistance vessels of wild-type and endothelial NO synthase knockout mice. Proc. Natl. Acad. Sci. U.S.A. 97, 9747–9752. 10.1073/pnas.97.17.9747 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

