speG Is Required for Intracellular Replication of Salmonella in Various Human Cells and Affects Its Polyamine Metabolism and Global Transcriptomes
- PMID: 29187844
- PMCID: PMC5694781
- DOI: 10.3389/fmicb.2017.02245
speG Is Required for Intracellular Replication of Salmonella in Various Human Cells and Affects Its Polyamine Metabolism and Global Transcriptomes
Abstract
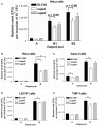
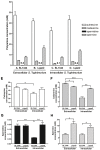
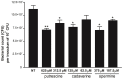
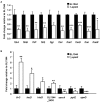
The speG gene has been reported to regulate polyamine metabolism in Escherichia coli and Shigella, but its role in Salmonella remains unknown. Our preliminary studies have revealed that speG widely affects the transcriptomes of infected in vitro M and Caco-2 cells and that it is required for the intracellular replication of Salmonella enterica serovar Typhimurium (S. Typhimurium) in HeLa cells. In this study, we demonstrated that speG plays a time-dependent and cell type-independent role in the intracellular replication of S. Typhimurium. Moreover, high-performance liquid chromatography (HPLC) of four major polyamines demonstrated putrescine, spermine, and cadaverine as the leading polyamines in S. Typhimurium. The deletion of speG significantly increased the levels of the three polyamines in intracellular S. Typhimurium, suggesting the inhibitory effect of speG on the biosynthesis of these polyamines. The deletion of speG was associated with elevated levels of these polyamines in the attenuated intracellular replication of S. Typhimurium in host cells. This result was subsequently validated by the dose-dependent suppression of intracellular proliferation after the addition of the polyamines. Furthermore, our RNA transcriptome analysis of S. Typhimurium SL1344 and its speG mutant outside and inside Caco-2 cells revealed that speG regulates the genes associated with flagellar biosynthesis, fimbrial expression, and functions of types III and I secretion systems. speG also affects the expression of genes that have been rarely reported to correlate with polyamine metabolism in Salmonella, including those associated with the periplasmic nitrate reductase system, glucarate metabolism, the phosphotransferase system, cytochromes, and the succinate reductase complex in S. Typhimurium in the mid-log growth phase, as well as those in the ilv-leu and histidine biosynthesis operons of intracellular S. Typhimurium after invasion in Caco-2 cells. In the present study, we characterized the phenotypes and transcriptome effects of speG in S. Typhimurium and reviewed the relevant literature to facilitate a more comprehensive understanding of the potential role of speG in the polyamine metabolism and virulence regulation of Salmonella.
Keywords: RNA microarray; Salmonella Typhimurium; flagella; intracellular replication; motility; polyamine; speG; transcriptome.
Figures


Similar articles
-
Impacts of Salmonella enterica Serovar Typhimurium and Its speG Gene on the Transcriptomes of In Vitro M Cells and Caco-2 Cells.PLoS One. 2016 Apr 11;11(4):e0153444. doi: 10.1371/journal.pone.0153444. eCollection 2016. PLoS One. 2016. PMID: 27064787 Free PMC article.
-
Polyamines are required for virulence in Salmonella enterica serovar Typhimurium.PLoS One. 2012;7(4):e36149. doi: 10.1371/journal.pone.0036149. Epub 2012 Apr 30. PLoS One. 2012. PMID: 22558361 Free PMC article.
-
Polyamine depletion has global effects on stress and virulence gene expression and affects HilA translation in Salmonella enterica serovar typhimurium.Res Microbiol. 2020 Apr-Jun;171(3-4):143-152. doi: 10.1016/j.resmic.2019.12.001. Epub 2020 Jan 25. Res Microbiol. 2020. PMID: 31991172
-
Effects of colonization-associated gene yqiC on global transcriptome, cellular respiration, and oxidative stress in Salmonella Typhimurium.J Biomed Sci. 2022 Dec 1;29(1):102. doi: 10.1186/s12929-022-00885-0. J Biomed Sci. 2022. PMID: 36457101 Free PMC article.
-
Molecular Profiling: Catecholamine Modulation of Gene Expression in Escherichia coli O157:H7 and Salmonella enterica Serovar Typhimurium.Adv Exp Med Biol. 2016;874:167-82. doi: 10.1007/978-3-319-20215-0_7. Adv Exp Med Biol. 2016. PMID: 26589218 Review.
Cited by
-
Organoid and Enteroid Modeling of Salmonella Infection.Front Cell Infect Microbiol. 2018 Apr 4;8:102. doi: 10.3389/fcimb.2018.00102. eCollection 2018. Front Cell Infect Microbiol. 2018. PMID: 29670862 Free PMC article. Review.
-
Structural and Kinetic Characterization of the SpeG Spermidine/Spermine N-acetyltransferase from Methicillin-Resistant Staphylococcus aureus USA300.Cells. 2023 Jul 12;12(14):1829. doi: 10.3390/cells12141829. Cells. 2023. PMID: 37508494 Free PMC article.
-
New Insights on the Early Interaction Between Typhoid and Non-typhoid Salmonella Serovars and the Host Cells.Front Microbiol. 2021 Jul 1;12:647044. doi: 10.3389/fmicb.2021.647044. eCollection 2021. Front Microbiol. 2021. PMID: 34276584 Free PMC article. Review.
-
SpeG polyamine acetyltransferase enzyme from Bacillus thuringiensis forms a dodecameric structure and exhibits high catalytic efficiency.J Struct Biol. 2020 Jun 1;210(3):107506. doi: 10.1016/j.jsb.2020.107506. Epub 2020 Apr 10. J Struct Biol. 2020. PMID: 32283314 Free PMC article.
-
The influence of the polyamine synthesis pathways on Pseudomonas syringae virulence and plant interaction.Microbiology (Reading). 2025 Jun;171(6):001569. doi: 10.1099/mic.0.001569. Microbiology (Reading). 2025. PMID: 40493022 Free PMC article.
References
-
- Calderon P. F., Morales E. H., Acuna L. G., Fuentes D. N., Gil F., Porwollik S., et al. . (2014). The small RNA RyhB homologs from Salmonella typhimurium participate in the response to S-nitrosoglutathione-induced stress. Biochem. Biophys. Res. Commun. 450, 641–645. 10.1016/j.bbrc.2014.06.031 - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

