Modulation of P2X7 Receptor during Inflammation in Multiple Sclerosis
- PMID: 29187851
- PMCID: PMC5694754
- DOI: 10.3389/fimmu.2017.01529
Modulation of P2X7 Receptor during Inflammation in Multiple Sclerosis
Abstract
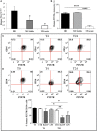
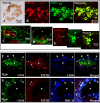
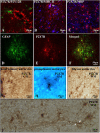
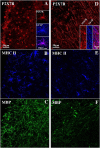
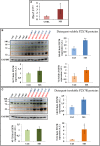
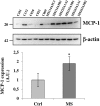
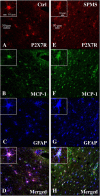
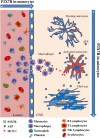
Multiple sclerosis (MS) is characterized by macrophage accumulation and inflammatory infiltrates into the CNS contributing to demyelination. Because purinergic P2X7 receptor (P2X7R) is known to be abundantly expressed on cells of the hematopoietic lineage and of the nervous system, we further investigated its phenotypic expression in MS and experimental autoimmune encephalomyelitis conditions. By quantitative reverse transcription polymerase chain reaction and flow cytometry, we analyzed the P2X7R expression in human mononuclear cells of peripheral blood from stable and acute relapsing-remitting MS phases. Human monocytes were also challenged in vitro with pro-inflammatory stimuli such as the lipopolysaccharide, or the P2X7R preferential agonist 2'(3')-O-(4 Benzoylbenzoyl)adenosine 5'-triphosphate, before evaluating P2X7R protein expression. Finally, by immunohistochemistry and immunofluorescence confocal analysis, we investigated the P2X7R expression in frontal cortex from secondary progressive MS cases. We demonstrated that P2X7R is present and inhibited on peripheral monocytes isolated from MS donors during the acute phase of the disease, moreover it is down-regulated in human monocytes after pro-inflammatory stimulation in vitro. P2X7R is instead up-regulated on astrocytes in the parenchyma of frontal cortex from secondary progressive MS patients, concomitantly with monocyte chemoattractant protein-1 chemokine, while totally absent from microglia/macrophages or oligodendrocytes, despite the occurrence of inflammatory conditions. Our results suggest that inhibition of P2X7R on monocytes and up-regulation in astrocytes might contribute to sustain inflammatory mechanisms in MS. By acquiring further knowledge about P2X7R dynamics and identifying P2X7R as a potential marker for the disease, we expect to gain insights into the molecular pathways of MS.
Keywords: P2X7 receptor; astrocytes; monocyte chemoattractant protein-1; monocytes; multiple sclerosis; neuroinflammation.
Figures

Similar articles
-
Two Single Nucleotide Polymorphisms in the Purinergic Receptor P2X7 Gene Are Associated with Disease Severity in Multiple Sclerosis.Int J Mol Sci. 2022 Dec 6;23(23):15381. doi: 10.3390/ijms232315381. Int J Mol Sci. 2022. PMID: 36499708 Free PMC article.
-
Purinergic receptors P2Y12R and P2X7R: potential targets for PET imaging of microglia phenotypes in multiple sclerosis.J Neuroinflammation. 2017 Dec 22;14(1):259. doi: 10.1186/s12974-017-1034-z. J Neuroinflammation. 2017. PMID: 29273052 Free PMC article.
-
Monocytes P2X7 purinergic receptor is modulated by glatiramer acetate in multiple sclerosis.J Neuroimmunol. 2012 Apr;245(1-2):93-7. doi: 10.1016/j.jneuroim.2012.02.002. Epub 2012 Feb 26. J Neuroimmunol. 2012. PMID: 22370183
-
The role of vitamin D and P2X7R in multiple sclerosis.J Neuroimmunol. 2019 May 15;330:159-169. doi: 10.1016/j.jneuroim.2019.03.004. Epub 2019 Mar 14. J Neuroimmunol. 2019. PMID: 30908981 Review.
-
Astroglial and Microglial Purinergic P2X7 Receptor as a Major Contributor to Neuroinflammation during the Course of Multiple Sclerosis.Int J Mol Sci. 2021 Aug 5;22(16):8404. doi: 10.3390/ijms22168404. Int J Mol Sci. 2021. PMID: 34445109 Free PMC article. Review.
Cited by
-
Purinergic Receptors of the Central Nervous System: Biology, PET Ligands, and Their Applications.Mol Imaging. 2020 Jan-Dec;19:1536012120927609. doi: 10.1177/1536012120927609. Mol Imaging. 2020. PMID: 32539522 Free PMC article. Review.
-
Role of P2X7 Receptors in Immune Responses During Neurodegeneration.Front Cell Neurosci. 2021 May 26;15:662935. doi: 10.3389/fncel.2021.662935. eCollection 2021. Front Cell Neurosci. 2021. PMID: 34122013 Free PMC article. Review.
-
Empowering the NSC-34 cell line as a motor neuron model: Cytosine arabinoside's action.Neural Regen Res. 2026 Jan 1;21(1):357-364. doi: 10.4103/NRR.NRR-D-24-00034. Epub 2024 Sep 24. Neural Regen Res. 2026. PMID: 39314144 Free PMC article.
-
Pleiotropic Roles of P2X7 in the Central Nervous System.Front Cell Neurosci. 2019 Sep 4;13:401. doi: 10.3389/fncel.2019.00401. eCollection 2019. Front Cell Neurosci. 2019. PMID: 31551714 Free PMC article. Review.
-
Two Single Nucleotide Polymorphisms in the Purinergic Receptor P2X7 Gene Are Associated with Disease Severity in Multiple Sclerosis.Int J Mol Sci. 2022 Dec 6;23(23):15381. doi: 10.3390/ijms232315381. Int J Mol Sci. 2022. PMID: 36499708 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

