A Role for Iodide and Thyroglobulin in Modulating the Function of Human Immune Cells
- PMID: 29187856
- PMCID: PMC5694785
- DOI: 10.3389/fimmu.2017.01573
A Role for Iodide and Thyroglobulin in Modulating the Function of Human Immune Cells
Abstract
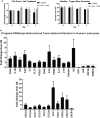
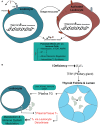
Iodine is an essential element required for the function of all organ systems. Although the importance of iodine in thyroid hormone synthesis and reproduction is well known, its direct effects on the immune system are elusive. Human leukocytes expressed mRNA of iodide transporters (NIS and PENDRIN) and thyroid-related proteins [thyroglobulin (TG) and thyroid peroxidase (TPO)]. The mRNA levels of PENDRIN and TPO were increased whereas TG transcripts were decreased post leukocyte activation. Flow cytometric analysis revealed that both PENDRIN and NIS were expressed on the surface of leukocyte subsets with the highest expression occurring on monocytes and granulocytes. Treatment of leukocytes with sodium iodide (NaI) resulted in significant changes in immunity-related transcriptome with an emphasis on increased chemokine expression as probed with targeted RNASeq. Similarly, treatment of leukocytes with NaI or Lugol's iodine induced increased protein production of both pro- and anti-inflammatory cytokines. These alterations were not attributed to iodide-induced de novo thyroid hormone synthesis. However, upon incubation with thyroid-derived TG, primary human leukocytes but not Jurkat T cells released thyroxine and triiodothyronine indicating that immune cells could potentially influence thyroid hormone balance. Overall, our studies reveal the novel network between human immune cells and thyroid-related molecules and highlight the importance of iodine in regulating the function of human immune cells.
Keywords: NIS; RNAseq; iodine; iodine deficiency; nutritional immunology; pendrin; thyroglobulin; thyroid hormones.
Figures



Similar articles
-
Correlation between the loss of thyroglobulin iodination and the expression of thyroid-specific proteins involved in iodine metabolism in thyroid carcinomas.J Clin Endocrinol Metab. 2003 Oct;88(10):4977-83. doi: 10.1210/jc.2003-030586. J Clin Endocrinol Metab. 2003. PMID: 14557483
-
Escape from the acute Wolff-Chaikoff effect is associated with a decrease in thyroid sodium/iodide symporter messenger ribonucleic acid and protein.Endocrinology. 1999 Aug;140(8):3404-10. doi: 10.1210/endo.140.8.6893. Endocrinology. 1999. PMID: 10433193
-
Sodium/iodide symporter (NIS) gene expression is the limiting step for the onset of thyroid function in the human fetus.J Clin Endocrinol Metab. 2007 Jan;92(1):70-6. doi: 10.1210/jc.2006-1450. Epub 2006 Oct 31. J Clin Endocrinol Metab. 2007. PMID: 17077129
-
Update on intrathyroidal iodine metabolism.Thyroid. 2001 May;11(5):407-14. doi: 10.1089/105072501300176363. Thyroid. 2001. PMID: 11396699 Review.
-
Iodine-Induced hypothyroidism.Thyroid. 2001 May;11(5):501-10. doi: 10.1089/105072501300176462. Thyroid. 2001. PMID: 11396709 Review.
Cited by
-
In vivo anti-V-ATPase antibody treatment delays ovarian tumor growth by increasing antitumor immune responses.Mol Oncol. 2020 Oct;14(10):2436-2454. doi: 10.1002/1878-0261.12782. Epub 2020 Sep 10. Mol Oncol. 2020. PMID: 32797726 Free PMC article.
-
The Prevalence and Risk Factors Associated with Iodine Deficiency in Canadian Adults.Nutrients. 2022 Jun 21;14(13):2570. doi: 10.3390/nu14132570. Nutrients. 2022. PMID: 35807751 Free PMC article.
-
Dietary mineral intakes predict Coronavirus-disease 2019 (COVID-19) incidence and hospitalization in older adults.BMC Nutr. 2024 Mar 4;10(1):42. doi: 10.1186/s40795-024-00821-5. BMC Nutr. 2024. PMID: 38439106 Free PMC article.
-
Iodine loaded nanoparticles with commercial applicability increase survival in mice cancer models with low degree of side effects.Cancer Rep (Hoboken). 2023 Aug;6(8):e1843. doi: 10.1002/cnr2.1843. Epub 2023 Jun 2. Cancer Rep (Hoboken). 2023. PMID: 37269144 Free PMC article.
-
Beneficial Effects of Vitamins, Minerals, and Bioactive Peptides on Strengthening the Immune System Against COVID-19 and the Role of Cow's Milk in the Supply of These Nutrients.Biol Trace Elem Res. 2022 Nov;200(11):4664-4677. doi: 10.1007/s12011-021-03045-x. Epub 2021 Nov 27. Biol Trace Elem Res. 2022. PMID: 34837602 Free PMC article. Review.
References
-
- Rousset B, Dupuy C, Miot F, Dumont J. Thyroid hormone synthesis and secretion. In: De Groot LJ, Chrousos G, Dungan K, Feingold KR, Grossman A, Hershman JM, Koch C, Korbonits M, McLachlan R, New M, Purnell J, Rebar R, Singer F, Vinik A, editors. Endotext. (Chap. 2), South Dartmouth, MA: (2000). p. 2–3. Available from: http://www.thyroidmanager.org/wp-content/uploads/chapters/chapter-2-thyr...
-
- Benoist BD, Andersson M, Egli I, Takkouche B, Allen H. Iodine Status Worldwide. Geneva: World Health Organization; (2004).
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

