Long non-coding RNA UICLM promotes colorectal cancer liver metastasis by acting as a ceRNA for microRNA-215 to regulate ZEB2 expression
- PMID: 29187907
- PMCID: PMC5706103
- DOI: 10.7150/thno.20942
Long non-coding RNA UICLM promotes colorectal cancer liver metastasis by acting as a ceRNA for microRNA-215 to regulate ZEB2 expression
Erratum in
-
Erratum: Long non-coding RNA UICLM promotes colorectal cancer liver metastasis by acting as a ceRNA for microRNA-215 to regulate ZEB2 expression: Erratum.Theranostics. 2025 Jan 9;15(5):2086-2087. doi: 10.7150/thno.108968. eCollection 2025. Theranostics. 2025. PMID: 39897561 Free PMC article.
Abstract
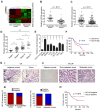
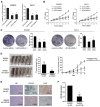
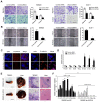
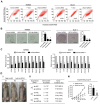
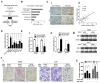
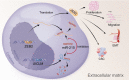
Long non-coding RNAs (lncRNAs) are involved in the pathology of various tumors, including colorectal cancer (CRC). However, the role of lncRNA in CRC liver metastasis remains unclear. Methods: a microarray was performed to identify the differentially expressed lncRNAs between CRC tissues with and without liver metastasis. Survival analysis was evaluated using the Kaplan-Meier method and assessed using the log-rank test. In vitro and in vivo assays were preformed to explore the biological effects of the differentially expressed lncRNA in CRC cells. Results: the lncRNA UICLM (up-regulated in colorectal cancer liver metastasis) was significantly up-regulated in cases of CRC with liver metastasis. Moreover, UICLM expression was higher in CRC tissues than in normal tissues, and UICLM expression was associated with poor patient survival. Knockdown of UICLM inhibited CRC cell proliferation, invasion, epithelial-mesenchymal transition (EMT) and CRC stem cell formation in vitro as well as tumor growth and liver metastasis in vivo. Ectopic expression of UICLM promoted CRC cell proliferation and invasion. Mechanistic investigations revealed that UICLM induced its biological effects by regulating ZEB2, as the oncogenesis facilitated by UICLM was inhibited by ZEB2 depletion. Further study indicated that UICLM acted as a competing endogenous RNA (ceRNA) for miR-215 to regulate ZEB2 expression. Conclusions: taken together, our findings demonstrate how UICLM induces CRC liver metastasis and may offer a novel prognostic marker and therapeutic target for this disease.
Keywords: CRC; liver metastasis.; lncRNA UICLM; long non-coding RNA.
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures

References
-
- Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108. - PubMed
-
- Chang GJ, Rodriguez-Bigas MA, Skibber JM, Moyer VA. Lymph node evaluation and survival after curative resection of colon cancer: systematic review. J Natl Cancer Inst. 2007;99:433–41. - PubMed
-
- Esteller M. Non-coding RNAs in human disease. Nat Rev Genet. 2011;12:861–74. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

