Oval Cells Contribute to Fibrogenesis of Marginal Liver Grafts under Stepwise Regulation of Aldose Reductase and Notch Signaling
- PMID: 29187911
- PMCID: PMC5706107
- DOI: 10.7150/thno.20085
Oval Cells Contribute to Fibrogenesis of Marginal Liver Grafts under Stepwise Regulation of Aldose Reductase and Notch Signaling
Abstract
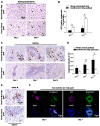
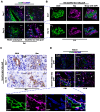
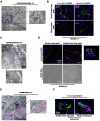
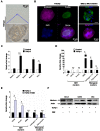
Background and Aims: Expanded donor criteria poses increased risk for late phase complications such as fibrosis that lead to graft dysfunction in liver transplantation. There remains a need to elucidate the precise mechanisms of post-transplant liver damage in order to improve the long-term outcomes of marginal liver grafts. In this study, we aimed to examine the role of oval cells in fibrogenic development of marginal liver grafts and explore the underlying mechanisms. Methods: Using an orthotopic rat liver transplantation model and human post-transplant liver biopsy tissues, the dynamics of oval cells in marginal liver grafts was evaluated by the platform integrating immuno-labeling techniques and ultrastructure examination. Underlying mechanisms were further explored in oval cells and an Aldose reductase (AR) knockout mouse model simulating marginal graft injury. Results: We demonstrated that activation of aldose reductase initiated oval cell proliferation in small-for-size fatty grafts during ductular reaction at the early phase after transplantation. These proliferative oval cells subsequently showed prevailing biliary differentiation and exhibited features of mesenchymal transition including dynamically co-expressing epithelial and mesenchymal markers, developing microstructures for extra-cellular matrix degradation (podosomes) or cell migration (filopodia and blebs), and acquiring the capacity in collagen production. Mechanistic studies further indicated that transition of oval cell-derived biliary cells toward mesenchymal phenotype ensued fibrogenesis in marginal grafts under the regulation of notch signaling pathway. Conclusions: Oval cell activation and their subsequent lineage commitment contribute to post-transplant fibrogenesis of small-for-size fatty liver grafts. Interventions targeting oval cell dynamics may serve as potential strategies to refine current clinical management.
Keywords: aldose reductase; hepatic bipotent cells; notch signaling.; small-for-size fatty graft injury.
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures



References
-
- Peeters PM, Sieders E, vd Heuvel M, Bijleveld CM, de Jong KP, TenVergert EM, Slooff MJ, Gouw AS. Predictive factors for portal fibrosis in pediatric liver transplant recipients. Transplantation. 2000;70:1581–7. - PubMed
-
- Imber CJ, St Peter SD, Handa A, Friend PJ. Hepatic steatosis and its relationship to transplantation. Liver Transpl. 2002;8:415–23. - PubMed
-
- Man K, Lo CM, Ng IO, Wong YC, Qin LF, Fan ST, Wong J. Liver transplantation in rats using small-for-size grafts: a study of hemodynamic and morphological changes. Arch Surg. 2001;136:280–5. - PubMed
-
- Cheng Q, Ng KT, Fan ST, Lim ZX, Guo DY, Liu XB, Liu Y, Poon RT, Lo CM, Man K. Distinct mechanism of small-for-size fatty liver graft injury-Wnt4 signaling activates hepatic stellate cells. Am J Transplant. 2010;10:1178–88. - PubMed
-
- Beckebaum S, Iacob S, Klein CG, Dechene A, Varghese J, Baba HA, Sotiropoulos GC, Paul A, Gerken G, Cicinnati VR. Assessment of allograft fibrosis by transient elastography and noninvasive biomarker scoring systems in liver transplant patients. Transplantation. 2010;89:983–93. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

