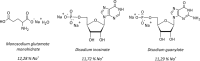Monosodium glutamate as a tool to reduce sodium in foodstuffs: Technological and safety aspects
- PMID: 29188030
- PMCID: PMC5694874
- DOI: 10.1002/fsn3.499
Monosodium glutamate as a tool to reduce sodium in foodstuffs: Technological and safety aspects
Abstract
Sodium chloride (NaCl) is the most commonly used ingredient to provide salty taste to foods. However, excess sodium in the bloodstream has been associated with the development of several chronic noncommunicable diseases. In order to limit sodium intake to levels considered safe, the World Health Organization (WHO) recommends for adults a daily intake of not more than 5 g of NaCl (less than 2 g of sodium). One of the strategic actions recommended by the Pan American Health Organization (PAHO) to reduce sodium intake is reformulation of processed foods. This recommendation indicates there is an urgent need to find salt substitutes, and umami compounds have been pointed as an alternative strategy. Like salty, umami is also a basic taste and the major compound associated to umami is monosodium L-glutamate (MSG). The available scientific data on the toxicity of MSG has been evaluated by scientific committees and regulatory agencies. The Joint FAO/WHO Expert Committee on Food Additives and the Scientific Committee on Food of the European Commission established an acceptable daily intake (ADI) not specified, which indicated that the substance offers no health risk when used as a food additive. The United States Food and Drug Administration and the Federation of American Societies for Experimental Biology classified MSG as a Generally Recognized as Safe (GRAS) substance. In this paper, an overview about salty and umami taste physiology, the potential applications of MSG use to reduce sodium content in specific industrialized foods and safety aspects of MSG as food additive are presented.
Keywords: Monosodium L‐glutamate; salty taste; sodium chloride; umami taste.
Figures
Similar articles
-
Effect of Monosodium Glutamate on Saltiness and Palatability Ratings of Low-Salt Solutions in Japanese Adults According to Their Early Salt Exposure or Salty Taste Preference.Nutrients. 2021 Feb 9;13(2):577. doi: 10.3390/nu13020577. Nutrients. 2021. PMID: 33572364 Free PMC article.
-
Glutamate-Sodium Discrimination Status in Adults Is Associated with Salt Recognition Threshold and Habitual Intake of Discretionary Food and Meat: A Cross-Sectional Study.Int J Environ Res Public Health. 2022 Sep 5;19(17):11101. doi: 10.3390/ijerph191711101. Int J Environ Res Public Health. 2022. PMID: 36078816 Free PMC article.
-
Females' ability to discriminate MSG from NaCl influences perceived intensity but not liking of MSG added vegetable broths.J Food Sci. 2020 Nov;85(11):3934-3942. doi: 10.1111/1750-3841.15478. Epub 2020 Oct 10. J Food Sci. 2020. PMID: 33037609
-
The safety evaluation of monosodium glutamate.J Nutr. 2000 Apr;130(4S Suppl):1049S-52S. doi: 10.1093/jn/130.4.1049S. J Nutr. 2000. PMID: 10736380 Review.
-
L-glutamate: a key amino acid for senory and metabolic functions.Arch Latinoam Nutr. 2016 Jun;66(2):101-112. Arch Latinoam Nutr. 2016. PMID: 29737666 Review.
Cited by
-
Nuclear magnetic resonance-based metabolomics analysis and characteristics of beef in different fattening periods.J Anim Sci Technol. 2020 May;62(3):321-333. doi: 10.5187/jast.2020.62.3.321. Epub 2020 May 31. J Anim Sci Technol. 2020. PMID: 32568257 Free PMC article.
-
Enhancing saltiness perception by chemosensory interaction: an fMRI study.Sci Rep. 2023 Jul 10;13(1):11128. doi: 10.1038/s41598-023-38137-2. Sci Rep. 2023. PMID: 37429921 Free PMC article.
-
Is L-Glutamate Toxic to Neurons and Thereby Contributes to Neuronal Loss and Neurodegeneration? A Systematic Review.Brain Sci. 2022 Apr 29;12(5):577. doi: 10.3390/brainsci12050577. Brain Sci. 2022. PMID: 35624964 Free PMC article. Review.
-
Sensory Flavor Profile of Split Gill Mushroom (Schizophyllum commune) Extract and Its Enhancement Effect on Taste Perception in Salt Solution and Seasoned Clear Soup.Foods. 2023 Oct 12;12(20):3745. doi: 10.3390/foods12203745. Foods. 2023. PMID: 37893641 Free PMC article.
-
High-pressure processing enhances saltiness perception and sensory acceptability of raw but not of cooked cured pork loins-leveraging salty and umami taste.Front Nutr. 2024 Feb 15;11:1352550. doi: 10.3389/fnut.2024.1352550. eCollection 2024. Front Nutr. 2024. PMID: 38425479 Free PMC article.
References
-
- Ainsworth, P. , & Plunkett, A. (2007). Reducing salt in snack products In Kilcast D., & Angus F. (Eds.), Reducing salt in foods – Practical strategies. Cambridge, England: Woodhead Publishing Limited.
-
- Allen, D. H. , Delohery, J. , & Baker, G. (1987). Monosodium L‐glutamate‐induced asthma. Journal of Allergy Clinical Immunology, 80(4), 530–537. - PubMed
-
- ANVISA. Agência Nacional de Vigilância Sanitária . (2001). Resolução da Diretoria Colegiada – RDC nº 1, de 2 de janeiro de 2001. Regulamento Técnico que aprova o uso de Aditivos com a função de Realçadores de Sabor, Estabelecendo seus Limites Máximos para os Alimentos. Available from http://portal.anvisa.gov.br/wps/wcm/connect/9a9d6200400ab428a70ae76d6e8a... (accessed 5 May 2016).
-
- ANVISA. Agência Nacional de Vigilância Sanitária . (2003). Resolução da Diretoria Colegiada ‐ RDC n. 359, de 23 de dezembro de 2003. Aprova Regulamento Técnico de Porções de Alimentos Embalados para Fins de Rotulagem Nutricional. Diário Oficial da União; Poder Executivo, de 26 de dezembro de 2003. Available from http://portal.anvisa.gov.br/wps/wcm/connect/d12c9e804745947f9bf0df3fbc4c... (accessed 5 May 2016).
-
- ANVISA. Agência Nacional de Vigilância Sanitária . (2010). Resolução da Diretoria Colegiada – RDC n. 45, de 03 de novembro de 2010. Dispõe sobre aditivos alimentares autorizados para uso segundo as Boas Práticas de Fabricação (BPF). Diário Oficial da União, Brasília. 2010 nov. 05: Seção 1, p. 68. Available from http://portal.anvisa.gov.br/wps/wcm/connect/11707300474597459fc3df3fbc4c.... (accessed 5 May 2016).
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources