Molecular cloning, expression, purification and functional characterization of an antifungal cyclophilin protein from Panax ginseng
- PMID: 29188056
- PMCID: PMC5702963
- DOI: 10.3892/br.2017.998
Molecular cloning, expression, purification and functional characterization of an antifungal cyclophilin protein from Panax ginseng
Abstract
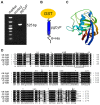
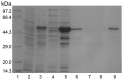
Cyclophilins (CyPs), a member of peptidyl-prolyl cis-trans isomerases (PPIases), are ubiquitously distributed in organisms such as bacteria, yeast, plants and animals. CyPs have diverse biological functions, with some exhibiting antifungal and antiviral activities. In this study, Panax ginseng cyclophilin (pgCyP), a novel gene encoding an antifungal protein from Panax ginseng, was cloned, and its protein product was expressed in Escherichia coli, and then fractionated by affinity chromatography. The open reading frame of the pgCyP full-length coding sequence was found to encode a single-domain CyP-like protein of 174 amino residues with a calculated molecular weight of 18.7 kDa. The pGEX system was used to express pgCyP fused to glutathione S-transferase. After affinity purification, the protein showed a strong fungal resistance effect on Phytophthora cactorum. In addition, pgCyP showed high PPIase activity. To the best of our knowledge, the present study is the first successful effort to clone and characterize a CyP-like protein gene from Panax ginseng.
Keywords: Panax ginseng; antifungal activity; cyclophilin; expression and purification; peptidyl-prolyl cis-trans isomerase.
Figures


Similar articles
-
Bioinformatics Analysis of the Panax ginseng Cyclophilin Gene and Its Anti-Phytophthora cactorum Activity.Plants (Basel). 2024 Sep 29;13(19):2731. doi: 10.3390/plants13192731. Plants (Basel). 2024. PMID: 39409601 Free PMC article.
-
Cloning and characterization of ppiB, a Bacillus subtilis gene which encodes a cyclosporin A-sensitive peptidyl-prolyl cis-trans isomerase.Mol Microbiol. 1994 Mar;11(6):1073-83. doi: 10.1111/j.1365-2958.1994.tb00384.x. Mol Microbiol. 1994. PMID: 8022278
-
Molecular cloning, recombinant expression, and antifungal functional characterization of the lipid transfer protein from Panax ginseng.Biotechnol Lett. 2016 Jul;38(7):1229-35. doi: 10.1007/s10529-016-2100-9. Epub 2016 Apr 6. Biotechnol Lett. 2016. PMID: 27053083
-
Archaeal peptidyl prolyl cis-trans isomerases (PPIases) update 2004.Front Biosci. 2004 May 1;9:1680-720. doi: 10.2741/1361. Front Biosci. 2004. PMID: 14977579 Review.
-
Archaeal peptidyl prolyl cis-trans isomerases (PPIases).Front Biosci. 2000 Sep 1;5:D821-36. doi: 10.2741/maruyama. Front Biosci. 2000. PMID: 10966874 Review.
Cited by
-
High-yield production of protopanaxadiol from sugarcane molasses by metabolically engineered Saccharomyces cerevisiae.Microb Cell Fact. 2022 Nov 5;21(1):230. doi: 10.1186/s12934-022-01949-4. Microb Cell Fact. 2022. PMID: 36335407 Free PMC article.
-
Genome-wide identification of cyclophilin genes in Gossypium hirsutum and functional characterization of a CYP with antifungal activity against Verticillium dahliae.BMC Plant Biol. 2019 Jun 21;19(1):272. doi: 10.1186/s12870-019-1848-1. BMC Plant Biol. 2019. PMID: 31226952 Free PMC article.
-
Plant Cyclophilins: Multifaceted Proteins With Versatile Roles.Front Plant Sci. 2020 Oct 22;11:585212. doi: 10.3389/fpls.2020.585212. eCollection 2020. Front Plant Sci. 2020. PMID: 33193535 Free PMC article. Review.
-
Bioinformatics Analysis of the Panax ginseng Cyclophilin Gene and Its Anti-Phytophthora cactorum Activity.Plants (Basel). 2024 Sep 29;13(19):2731. doi: 10.3390/plants13192731. Plants (Basel). 2024. PMID: 39409601 Free PMC article.
-
Cyclophilins and Their Functions in Abiotic Stress and Plant-Microbe Interactions.Biomolecules. 2021 Sep 21;11(9):1390. doi: 10.3390/biom11091390. Biomolecules. 2021. PMID: 34572603 Free PMC article. Review.
References
-
- Sattayasai N, Sudmoon R, Nuchadomrong S, Chaveerach A, Kuehnle AR, Mudalige-Jayawickrama RG, Bunyatratchata W. Dendrobium findleyanum agglutinin: Production, localization, anti-fungal activity and gene characterization. Plant Cell Rep. 2009;28:1243–1252. doi: 10.1007/s00299-009-0724-0. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
