Ultrahigh-resolution optical coherence elastography through a micro-endoscope: towards in vivo imaging of cellular-scale mechanics
- PMID: 29188108
- PMCID: PMC5695958
- DOI: 10.1364/BOE.8.005127
Ultrahigh-resolution optical coherence elastography through a micro-endoscope: towards in vivo imaging of cellular-scale mechanics
Abstract
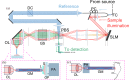
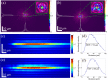
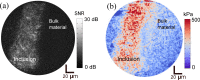
In this paper, we describe a technique capable of visualizing mechanical properties at the cellular scale deep in living tissue, by incorporating a gradient-index (GRIN)-lens micro-endoscope into an ultrahigh-resolution optical coherence elastography system. The optical system, after the endoscope, has a lateral resolution of 1.6 µm and an axial resolution of 2.2 µm. Bessel beam illumination and Gaussian mode detection are used to provide an extended depth-of-field of 80 µm, which is a 4-fold improvement over a fully Gaussian beam case with the same lateral resolution. Using this system, we demonstrate quantitative elasticity imaging of a soft silicone phantom containing a stiff inclusion and a freshly excised malignant murine pancreatic tumor. We also demonstrate qualitative strain imaging below the tissue surface on in situ murine muscle. The approach we introduce here can provide high-quality extended-focus images through a micro-endoscope with potential to measure cellular-scale mechanics deep in tissue. We believe this tool is promising for studying biological processes and disease progression in vivo.
Keywords: (110.4500) Optical coherence tomography; (170.2150) Endoscopic imaging; (170.3880) Medical and biological imaging.
Conflict of interest statement
BFK: OncoRes Medical (F,I), AC and DDS: OncoRes Medical (I). The other authors declare that there are no conflicts of interest related to this article.
Figures



References
-
- Butt H.-J., Cappella B., Kappl M., “Force measurements with the atomic force microscope: Technique, interpretation and applications,” Surf. Sci. Rep. 59(1–6), 1–152 (2005).10.1016/j.surfrep.2005.08.003 - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources
