Clinical and metabolic features of the randomised controlled Diabetes Remission Clinical Trial (DiRECT) cohort
- PMID: 29188339
- PMCID: PMC6448967
- DOI: 10.1007/s00125-017-4503-0
Clinical and metabolic features of the randomised controlled Diabetes Remission Clinical Trial (DiRECT) cohort
Abstract
Aims/hypothesis: Substantial weight loss in type 2 diabetes can achieve a return to non-diabetic biochemical status, without the need for medication. The Diabetes Remission Clinical Trial (DiRECT), a cluster-randomised controlled trial, is testing a structured intervention designed to achieve and sustain this over 2 years in a primary care setting to determine practicability for routine clinical practice. This paper reports the characteristics of the baseline cohort.
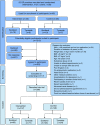
Methods: People with type 2 diabetes for <6 years with a BMI of 27-45 kg/m2 were recruited in 49 UK primary care practices, randomised to either best-practice diabetes care alone or with an additional evidence-based weight management programme (Counterweight-Plus). The co-primary outcomes, at 12 months, are weight loss ≥15 kg and diabetes remission (HbA1c <48 mmol/mol [6.5%]) without glucose-lowering therapy for at least 2 months. Outcome assessors are blinded to group assignment.
Results: Of 1510 people invited, 423 (28%) accepted; of whom, 306 (72%) were eligible at screening and gave informed consent. Seven participants were later found to have been randomised in error and one withdrew consent, leaving 298 (176 men, 122 women) who will form the intention to treat (ITT) population for analysis. Mean (SD) age was 54.4 (7.6) years, duration of diabetes 3.0 (1.7) years, BMI 34.6 (4.4) kg/m2 for all participants (34.2 (4.2) kg/m2 in men and 35.3 (4.6) kg/m2 in women) and baseline HbA1c (on treatment) 59.3 (12.7) mmol/mol (7.6% [1.2%]). The recruitment rate in the intervention and control groups, and comparisons between the subgroups recruited in Scotland and England, showed few differences.
Conclusions/interpretation: DiRECT has recruited a cohort of people with type 2 diabetes with characteristics similar to those seen in routine practice, indicating potential widespread applicability. Over 25% of the eligible population wished to participate in the study, including a high proportion of men, in line with the prevalence distribution of type 2 diabetes.
Trial registration: www.controlled-trials.com/ISRCTN03267836 ; date of registration 20 December 2013.
Keywords: Formula diet; Remission; Type 2 diabetes; Weight management.
Conflict of interest statement
Data availability
The datasets generated and/or analysed during the current study are available from the corresponding author on reasonable request.
Duality of interest
ML and NB have received funding from Cambridge Weight Plan for conference attendance and for other departmental research. Prior to study commencement, NB, HR and LMcC were shareholders and employees of Counterweight Ltd. HR and NB remain employees and shareholders of Counterweight Ltd. ML has provided consultancy to Counterweight Ltd.
Contribution statement
ML and RT conceived the study and are the principal investigators. All authors contributed to the design of the study. WL is the trial coordinator and coordinated recruitment and acquisition of study data. YMcI coordinated the recruitment of GP practices. NB, GT, LMcC and AB recruited participants and contributed to the acquisition of data. SK and IF managed the study data and AMcC performed the statistical analyses. PW and NS conducted the biochemical analyses. CP, SZ, KH, JM and AAM contributed to the acquisition, analyses and interpretation of mechanistic study data. HR, a grant holder on the study, provided expertise on the Counterweight-Plus programme delivery. FS, AR, LR and AA contributed to the acquisition, analyses and interpretation of qualitative data. ML, WL and RT drafted the manuscript. All authors critically reviewed and revised the manuscript, and have read and approved the final version. All authors agree to be accountable for all aspects of the work in respect of accuracy and integrity. Overall, ML and RT are the guarantors of this work, had full access to all study data, and take responsibility for the integrity of the data and the accuracy of the data analyses.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous