Inhibitory influence of natural flavonoids on human protein kinase CK2 isoforms: effect of the regulatory subunit
- PMID: 29188536
- PMCID: PMC6002439
- DOI: 10.1007/s11010-017-3228-1
Inhibitory influence of natural flavonoids on human protein kinase CK2 isoforms: effect of the regulatory subunit
Abstract
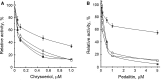
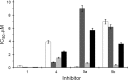
CK2 is a pleiotropic, constitutively active protein kinase responsible for the phosphorylation of more than 300 physiological substrates. Typically, this enzyme is found in tetrameric form consisting of two regulatory subunits CK2β and two catalytic subunits CK2α or CK2α'. Several natural occurring flavonoids were tested for their ability to inhibit both CK2 holoenzymes, CK2α2β2 and CK2α'2β2. We identified few substances selectively inhibiting only the α' subunit. Other compounds showed similar effect towards all four isoforms. In some cases, like chrysoeriol, pedalitin, apigenin, and luteolin, the α2β2 holoenzyme was at least six times better inhibited than the free α subunit. Otherwise, we have found a luteolin derivative decreased the kinase activity of CK2α' with an IC50 value of 0.8 μM, but the holoenzyme only with 9.5 µM.
Keywords: CK2 holoenzymes; Flavonoids; Inhibitors; Phosphorylation.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures


Similar articles
-
Toward selective CK2alpha and CK2alpha' inhibitors: Development of a novel whole-cell kinase assay by Autodisplay of catalytic CK2alpha'.J Pharm Biomed Anal. 2016 Mar 20;121:253-260. doi: 10.1016/j.jpba.2016.01.011. Epub 2016 Jan 8. J Pharm Biomed Anal. 2016. PMID: 26786382
-
Biochemical characterization of CK2alpha and alpha' paralogues and their derived holoenzymes: evidence for the existence of a heterotrimeric CK2alpha'-holoenzyme forming trimeric complexes.Mol Cell Biochem. 2008 Sep;316(1-2):37-47. doi: 10.1007/s11010-008-9824-3. Epub 2008 Jun 24. Mol Cell Biochem. 2008. PMID: 18574672
-
Selected flavonoid compounds as promising inhibitors of protein kinase CK2α and CK2α', the catalytic subunits of CK2.Phytochemistry. 2017 Apr;136:39-45. doi: 10.1016/j.phytochem.2016.12.018. Epub 2016 Dec 30. Phytochemistry. 2017. PMID: 28043654
-
Protein Kinase CK2α', More than a Backup of CK2α.Cells. 2023 Dec 14;12(24):2834. doi: 10.3390/cells12242834. Cells. 2023. PMID: 38132153 Free PMC article. Review.
-
Conformational plasticity of the catalytic subunit of protein kinase CK2 and its consequences for regulation and drug design.Biochim Biophys Acta. 2010 Mar;1804(3):484-92. doi: 10.1016/j.bbapap.2009.09.022. Epub 2009 Sep 28. Biochim Biophys Acta. 2010. PMID: 19796713 Review.
Cited by
-
Cytotoxic and anti-excitotoxic effects of selected plant and algal extracts using COMET and cell viability assays.Sci Rep. 2021 Apr 19;11(1):8512. doi: 10.1038/s41598-021-88089-8. Sci Rep. 2021. PMID: 33875747 Free PMC article.
-
Ugonin J Acts as a SARS-CoV-2 3C-like Protease Inhibitor and Exhibits Anti-inflammatory Properties.Front Pharmacol. 2021 Aug 26;12:720018. doi: 10.3389/fphar.2021.720018. eCollection 2021. Front Pharmacol. 2021. PMID: 34512347 Free PMC article.
-
CK2 and protein kinases of the CK1 superfamily as targets for neurodegenerative disorders.Front Mol Biosci. 2022 Oct 6;9:916063. doi: 10.3389/fmolb.2022.916063. eCollection 2022. Front Mol Biosci. 2022. PMID: 36275622 Free PMC article. Review.
-
Boosting immunity: synergistic antiviral effects of luteolin, vitamin C, magnesium and zinc against SARS-CoV-2 3CLpro.Biosci Rep. 2024 Aug 28;44(8):BSR20240617. doi: 10.1042/BSR20240617. Biosci Rep. 2024. PMID: 39045772 Free PMC article.
-
Bioactive phytoconstituents as potent inhibitors of casein kinase-2: dual implications in cancer and COVID-19 therapeutics.RSC Adv. 2022 Mar 10;12(13):7872-7882. doi: 10.1039/d1ra09339h. eCollection 2022 Mar 8. RSC Adv. 2022. PMID: 35424745 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

