Analytical ultracentrifugation in structural biology
- PMID: 29188538
- PMCID: PMC5899701
- DOI: 10.1007/s12551-017-0340-0
Analytical ultracentrifugation in structural biology
Abstract
Researchers in the field of structural biology, especially X-ray crystallography and protein nuclear magnetic resonance, are interested in knowing as much as possible about the state of their target protein in solution. Not only is this knowledge relevant to studies of biological function, it also facilitates determination of a protein structure using homogeneous monodisperse protein samples. A researcher faced with a new protein to study will have many questions even after that protein has been purified. Analytical ultracentrifugation (AUC) can provide all of this information readily from a small sample in a non-destructive way, without the need for labeling, enabling structure determination experiments without any wasting time and material on uncharacterized samples. In this article, I use examples to illustrate how AUC can contribute to protein structural analysis. Integrating information from a variety of biophysical experimental methods, such as X-ray crystallography, small angle X-ray scattering, electrospray ionization-mass spectrometry, AUC allows a more complete understanding of the structure and function of biomacromolecules.
Keywords: AUC; Crystallography; Interaction; Protein.
Conflict of interest statement
Conflicts of interest
Satoru Unzai declares that he has no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by the author.
Figures
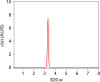
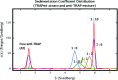

References
-
- Babitzke P (1997) Regulation of tryptophan biosynthesis: Trp-ing the TRAP or how Bacillus subtilis reinvented the wheel. Mol Microbiol 26:1–9 - PubMed
-
- Babitzke P (2004) Regulation of transcription attenuation and translation initiation by allosteric control of an RNA-binding protein: the Bacillus subtilis TRAP protein. Curr Opin Microbiol 7:132–139. 10.1016/j.mib.2004.02.003 - PubMed
-
- Babitzke P, Stults JT, Shire SJ, Yanofsky C (1994) TRAP, the trp RNA-binding attenuation protein of Bacillus subtilis, is a multisubunit complex that appears to recognize G/UAG repeats in the trpEDCFBA and trpG transcripts. J Biol Chem 269:16597–16604 - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

