Time-gated detection of protein-protein interactions with transcriptional readout
- PMID: 29189201
- PMCID: PMC5708895
- DOI: 10.7554/eLife.30233
Time-gated detection of protein-protein interactions with transcriptional readout
Abstract
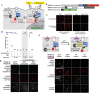
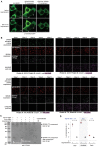
Transcriptional assays, such as yeast two-hybrid and TANGO, that convert transient protein-protein interactions (PPIs) into stable expression of transgenes are powerful tools for PPI discovery, screens, and analysis of cell populations. However, such assays often have high background and lose information about PPI dynamics. We have developed SPARK (Specific Protein Association tool giving transcriptional Readout with rapid Kinetics), in which proteolytic release of a membrane-tethered transcription factor (TF) requires both a PPI to deliver a protease proximal to its cleavage peptide and blue light to uncage the cleavage site. SPARK was used to detect 12 different PPIs in mammalian cells, with 5 min temporal resolution and signal ratios up to 37. By shifting the light window, we could reconstruct PPI time-courses. Combined with FACS, SPARK enabled 51 fold enrichment of PPI-positive over PPI-negative cells. Due to its high specificity and sensitivity, SPARK has the potential to advance PPI analysis and discovery.
Keywords: GPCR; LOV domain; TEV protease; Tango; cell biology; genetic selection; proximity-dependent labeling.
Conflict of interest statement
No competing interests declared.
AYT and WW have filed a patent application covering some aspects of this work.(U.S. Pat. App. No. 62/440,825).
Alice Y Ting: AYT and WW have filed a patent application covering some aspects of this work.(U.S. Pat. App. No. 62/440,825).
Figures






References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

