Routine antibiotic prophylaxis after normal vaginal birth for reducing maternal infectious morbidity
- PMID: 29190037
- PMCID: PMC6486135
- DOI: 10.1002/14651858.CD012137.pub2
Routine antibiotic prophylaxis after normal vaginal birth for reducing maternal infectious morbidity
Abstract
Background: Infectious morbidities contribute to considerable maternal and perinatal morbidity and mortality, including women at no apparent increased risk of infection. To reduce the incidence of infections, antibiotics are often administered to women after uncomplicated childbirth, particularly in settings where women are at higher risk of puerperal infectious morbidities.
Objectives: To assess whether routine administration of prophylactic antibiotics to women after normal (uncomplicated) vaginal birth, compared with placebo or no antibiotic prophylaxis, reduces postpartum maternal infectious morbidities and improves outcomes.
Search methods: We searched the Cochrane Pregnancy and Childbirth Group's Trials Register (31 August 2017), LILACS, ClinicalTrials.gov, the WHO International Clinical Trials Registry Platform (ICTRP) (22 August 2017) and reference lists of retrieved studies.
Selection criteria: We planned to include randomised or quasi-randomised trials evaluating the use of prophylactic antibiotics versus placebo or no antibiotic prophylaxis. Trials using a cluster-randomised design would have been eligible for inclusion, but we found none.In future updates of this review, we will include studies published in abstract form only, provided sufficient information is available to assess risks of bias. We will consider excluded abstracts for inclusion once the full publication is available, or the authors provide more information.Trials using a cross-over design are not eligible for inclusion in this review.
Data collection and analysis: Two review authors conducted independent assessment of trials for inclusion and risks of bias. They independently extracted data and checked them for accuracy, resolving differences in assessments by discussion. They evaluated methodological quality using standard Cochrane criteria and the GRADE approach.We present the summaries as risk ratios (RRs) and mean difference (MDs) using fixed- or random-effect models. For one primary outcome we found considerable heterogeneity and interaction. We explored further using subgroup analysis to investigate the effects of the randomisation unit. All review authors discussed and interpreted the results.
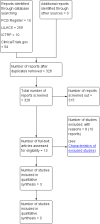
Main results: One randomised controlled trial (RCT) and two quasi-RCTs contributed data on 1779 women who had uncomplicated vaginal births, comparing different antibiotic regimens with placebo or no treatment. The included trials took place in the 1960s (one trial) and 1990s (two trials). The trials were conducted in France, the USA and Brazil. Antibiotics administered included: oral sulphamethoxypyridazine or chloramphenicol for three to five days, and intravenous amoxicillin and clavulanic acid in a single dose one hour after birth. We rated most of the domains for risk of bias as high risk, with the exception of reporting bias and other potential bias.The quality of evidence ranged from low to very low, based on the GRADE quality assessment, given very serious design limitations of the included studies, few events and wide confidence intervals (CIs) of effect estimates.We found a decrease in the risk of endometritis (RR 0.28, 95% CI 0.09 to 0.83, two trials, 1364 women,very low quality). However, one trial reported zero events for this outcome and we rate the evidence as very low quality. There was little or no difference between groups for the risk of urinary tract infection (RR 0.25, 95% CI 0.05 to 1.19, two trials, 1706 women,low quality), wound infection after episiotomy (reported as wound dehiscence in the included trials) (RR 0.78, 95% CI 0.31 to 1.96, two trials, 1364 women, very low quality) and length of maternal hospital stay in days (MD -0.15, 95% CI -0.31 to 0.01, one trial, 1291 women, very low quality). Cost of care in US dollar equivalent was 2½ times higher in the control group compared to the group receiving antibiotics prophylaxis (USD 3600: USD 9000, one trial, 1291 women). There were few or no differences between treated and control groups for adverse effects of antibiotics (skin rash) reported in one woman in each of the two trials (RR 3.03, 95% CI 0.32 to 28.95, two trials, 1706 women, very low quality). The incidence of severe maternal infectious morbidity, antimicrobial resistance or women's satisfaction with care were not addressed by any of the included studies.
Authors' conclusions: Routine administration of antibiotics may reduce the risk of endometritis after uncomplicated vaginal birth. The small number and nature of the trials limit the interpretation of the evidence for application in practice, particularly in settings where women may be at higher risk of developing endometritis. The use of antibiotics did not reduce the incidence of urinary tract infections, wound infection or the length of maternal hospital stay. Antibiotics are not a substitute for infection prevention and control measures around the time of childbirth and the postpartum period. The decision to routinely administer prophylactic antibiotics after normal vaginal births needs to be balanced by patient features, childbirth setting and provider experience, including considerations of the contribution of indiscriminate use of antibiotics to raising antimicrobial resistance. Well-designed and high-powered randomised controlled trials would help to evaluate the added value of routine antibiotic administration as a measure to prevent maternal infections after normal vaginal delivery.
Conflict of interest statement
Mercedes Bonet: none known. Erika Ota: none known. Chioma E Chibueze's work was financially supported by the The Grant of National Center for Child Health and Development, Japan 27B‐10, 26A‐5. Olufemi T Oladapo: none known.
Figures
Update of
References
References to studies included in this review
Fernandez 1993 {published data only}
-
- Fernandez H, Gagnepain A, Bourget P, Peray P, Frydman R, Papiernik E, et al. Antibiotic prophylaxis against postpartum endometritis after vaginal delivery: a prospective randomized comparison between Amox‐CA (Augmentin®) and abstention. European Journal of Obstetrics & Gynecology and Reproductive Biology 1993;50(3):169‐75. - PubMed
Neto 1990 {published data only}
-
- Neto S, Goncalves JA, Andrade LF. Clinical evaluation of the chloramphenicol use as a prophylactic antibiotic in the vaginal delivery with episiotomy [Avaliacao clinica do emprego do cloranfenicol como antibiotico profilatico no parto normal com episiotomia]. ACM: Arquivos Catarinenses de Medicina 1990;19(2):97‐102.
Turck 1962 {published data only}
-
- Turck M, Goffe BS, Petersdorf RG. Bacteriuria of pregnancy: relation to socioeconomic factors. New England Journal of Medicine 1962;266(17):857‐60. - PubMed
References to studies excluded from this review
Charles 1986 {published data only}
Cormier 1988 {published data only}
-
- Cormier P, Leng JJ, Janky E, Brouste V, Duthil B. Prevention using cefotetan of post‐partum and post‐abortion infectious complications in intra‐uterine procedures. Revue Francaise de Gynecologie et d'Obstetrique 1998;83(12):829‐32. - PubMed
Costa 1998 {published data only}
-
- Costa HF, Ávila ID, Gonçalves MM. Prophylactic antibiotic treatment in obstetrics: comparison of regimens. Revista Brasileira de Ginecologia e Obstetrícia 1998;20(9):509‐15.
Kampikaho 1993 {published data only}
-
- Kampikaho A, Irwig LM. A randomized trial of penicillin and streptomycin in the prevention of post‐partum infection in Uganda. International Journal of Gynecology & Obstetrics 1993;41(1):43‐52. - PubMed
Matthias 1986 {published data only}
-
- Mathias L, Kahhale S, Nestarez JE, Mathias AC, Cabaritti M. Prophylatic antibiotic in labor [Antibioticoterapia profilática no parto]. Arquivos Brasileiros de Medicina 1986;60(4):321‐4.
Oluwalana 2017 {published data only}
-
- Oluwalana C, Camara B, Bottomley C, Goodier S, Bojang A, Kampmann B, et al. Azithromycin in labor lowers clinical infections in mothers and newborns: a double‐blind trial. Pediatrics 2017;139(2):e20162281. - PubMed
Sharma 2014 {published data only}
-
- Sharma SK, Rogers BB, Alexander JM, McIntire DD, Leveno KJ. A randomized trial of the effects of antibiotic prophylaxis on epidural‐related fever in labor. Anesthesia & Analgesia 2014;118(3):604‐10. - PubMed
Yamagishi 2009 {published data only}
-
- Yamagishi Y, Izumi K, Tanaka K, Watanabe K, Mikamo H. Evaluation of antimicrobial prophylaxis after normal delivery. Japanese Journal of Antibiotics 2009;62(2):103‐15. - PubMed
Additional references
ACOG 2011
-
- American College of Obstetricians and Gynecologists. ACOG Practice Bulletin No. 120: Use of prophylactic antibiotics in labor and delivery. Obstetrics and Gynecology 2011;117(6):1472‐83. [PUBMED: 21606770] - PubMed
Acosta 2014
Dolea 2003
-
- Dolea C, Stein C. Global Burden of Disease 2000. Evidence and Information for Policy (EIP). Geneva: World Health Organization, 2003.
Higgins 2011
-
- Higgins JPT, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Hussein 2010
-
- Hussein J, Walker L. Chapter 8: Puerperal sepsis in low‐ and middle‐income settings: past, present and future. Maternal and Infant Deaths: Chasing Millennium Development Goals 4 & 5. London: RCOG Press, 2010:131‐47.
Hussein 2011
Khan 2006
-
- Khan KS, Wojdyla D, Say L, Gulmezoglu AM, Look PF. WHO analysis of causes of maternal death: a systematic review. Lancet 2006;367(9516):1066‐74. [PUBMED: 16581405] - PubMed
Mackeen 2015
Newton 2008
-
- Newton ER. Antibiotics in maternal‐fetal medicine. www.glowm.com/section_view/heading/Antibiotics%2520in%2520Maternal‐Fetal.... Available from, 2008 (accessed 31 October 2017). [DOI: 10.3843/GLOWM.10175] - DOI
Ngoc 2005
-
- Ngoc NT, Sloan NL, Thach TS, Liem le KB, Winikoff B. Incidence of postpartum infection after vaginal delivery in Viet Nam. Journal of Health, Population, and Nutrition 2005;23(2):121‐30. [PUBMED: 16117363] - PubMed
NICE 2014
-
- National Institute for Health and Care Excellence. Intrapartum care: care of healthy women and their babies during childbirth (Clinical Guideline 109). www.nice.org.uk/guidance/cg190 2014 (accessed 31 October 2017).
RevMan 2014 [Computer program]
-
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Say 2014
-
- Say L, Chou D, Gemmill A, Tunçalp Ö, Moller AB, Daniels J, et al. Global causes of maternal death: a WHO systematic analysis. Lancet. Global Health 2014;2(6):e323‐33. [PUBMED: 25103301] - PubMed
Sharma 2013
-
- Sharma M, Sanneving L, Mahadik K, Santacatterina M, Dhaneria S, Stålsby Lundborg C. Antibiotic prescribing in women during and after delivery in a non‐teaching, tertiary care hospital in Ujjain, India: a prospective cross‐sectional study. Journal of Pharmaceutical Policy and Practice 2013;6:9. [PUBMED: 25848538] - PMC - PubMed
Tharpe 2008
-
- Tharpe N. Postpregnancy genital tract and wound infections. Journal of Midwifery & Women's Health 2008;53(3):236‐46. [PUBMED: 18455098] - PubMed
Van Dillen 2010
-
- Dillen J, Zwart J, Schutte J, Roosmalen J. Maternal sepsis: epidemiology, etiology and outcome. Current Opinion in Infectious Diseases 2010;23(3):249‐54. [PUBMED: 20375891] - PubMed
WHO 1996
-
- World Health Organization. Care in normal birth: a practical guide. Report. Geneva: World Health Organization, 1996.
WHO 2005
-
- World Health Organization. The World Health Report 2005: Make every mother and child count. www.who.int/whr/2005/whr2005_en.pdf 2005 (accessed October 2015).
WHO 2014
-
- World Health Organization. Antimicrobial Resistance Global Report on Surveillance. Geneva: WHO, 2014.
References to other published versions of this review
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous