Distinct reaction mechanisms for hyaluronan biosynthesis in different kingdoms of life
- PMID: 29190396
- PMCID: PMC6192386
- DOI: 10.1093/glycob/cwx096
Distinct reaction mechanisms for hyaluronan biosynthesis in different kingdoms of life
Abstract
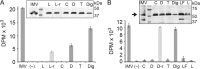
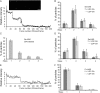
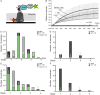
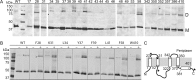
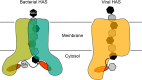
Hyaluronan (HA) is an acidic high molecular weight cell surface polysaccharide ubiquitously expressed by vertebrates, some pathogenic bacteria and even viruses. HA modulates many essential physiological processes and is implicated in numerous pathological conditions ranging from autoimmune diseases to cancer. In various pathogens, HA functions as a non-immunogenic surface polymer that reduces host immune responses. It is a linear polymer of strictly alternating glucuronic acid and N-acetylglucosamine units synthesized by HA synthase (HAS), a membrane-embedded family-2 glycosyltransferase. The enzyme synthesizes HA and secretes the polymer through a channel formed by its own membrane-integrated domain. To reveal how HAS achieves these tasks, we determined the biologically functional units of bacterial and viral HAS in a lipid bilayer environment by co-immunoprecipitation, single molecule fluorescence photobleaching, and site-specific cross-linking analyses. Our results demonstrate that bacterial HAS functions as an obligate homo-dimer with two functional HAS copies required for catalytic activity. In contrast, the viral enzyme, closely related to vertebrate HAS, functions as a monomer. Using site-specific cross-linking, we identify the dimer interface of bacterial HAS and show that the enzyme uses a reaction mechanism distinct from viral HAS that necessitates a dimeric assembly.
Keywords: hyaluronan; in vitro reconstitution; membrane transport; oligomeric state; polysaccharide.
© The Author(s) 2017. Published by Oxford University Press. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com.
Figures


References
-
- Bart G, Vico NO, Hassinen A, Pujol FM, Deen AJ, Ruusala A, Tammi RH, Squire A, Heldin P, Kellokumpu S et al. . 2015. Fluorescence resonance energy transfer (FRET) and proximity ligation assays reveal functionally relevant homo- and heteromeric complexes among hyaluronan synthases HAS1, HAS2, and HAS3. J Biol Chem. 290:11479–11490. - PMC - PubMed
-
- Bodevin-Authelet S, Kusche-Gullberg M, Pummill PE, DeAngelis PL, Lindahl U. 2005. Biosynthesis of hyaluronan: Direction of chain elongation. J Biol Chem. 280:8813–8818. - PubMed
-
- Camenisch TD, Spicer AP, Brehm-Gibson T, Biesterfeldt J, Augustine ML, Calabro A Jr, Kubalak S, Klewer SE, McDonald JA. 2000. Disruption of hyaluronan synthase-2 abrogates normal cardiac morphogenesis and hyaluronan-mediated transformation of epithelium to mesenchyme. J Clin Invest. 106:349–360. - PMC - PubMed
-
- Carter G, Annau E. 1953. Isolation of capsular polysaccharides from colonial variants of Pasteurella multocida. Am J Vet Res. 14:475–478. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

