BIGH3 Promotes Osteolytic Lesions in Renal Cell Carcinoma Bone Metastasis by Inhibiting Osteoblast Differentiation
- PMID: 29190493
- PMCID: PMC5711998
- DOI: 10.1016/j.neo.2017.11.002
BIGH3 Promotes Osteolytic Lesions in Renal Cell Carcinoma Bone Metastasis by Inhibiting Osteoblast Differentiation
Abstract
Background: Bone metastasis is common in renal cell carcinoma (RCC), and the lesions are mainly osteolytic. The mechanism of bone destruction in RCC bone metastasis is unknown.
Methods: We used a direct intrafemur injection of mice with bone-derived 786-O RCC cells (Bo-786) as an in vivo model to study if inhibition of osteoblast differentiation is involved in osteolytic bone lesions in RCC bone metastasis.
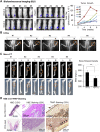
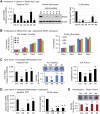
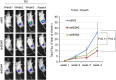
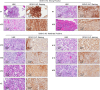
Results: We showed that bone-derived Bo-786 cells induced osteolytic bone lesions in the femur of mice. We examined the effect of conditioned medium of Bo-786 cells (Bo-786 CM) on both primary mouse osteoblasts and MC3T3-E1 preosteoblasts and found that Bo-786 CM inhibited osteoblast differentiation. Secretome analysis of Bo-786 CM revealed that BIGH3 (Beta ig h3 protein), also known as TGFBI (transforming growth factor beta-induced protein), is highly expressed. We generated recombinant BIGH3 and found that BIGH3 inhibited osteoblast differentiation in vitro. In addition, CM from Bo-786 BIGH3 knockdown cells (786-BIGH3 KD) reduced the inhibition of osteoblast differentiation compared to CM from vector control. Intrafemural injection of mice with 786-BIGH3 KD cells showed a reduction in osteolytic bone lesions compared to vector control. Immunohistochemical staining of 18 bone metastasis specimens from human RCC showed strong BIGH3 expression in 11/18 (61%) and moderate BIGH3 expression in 7/18 (39%) of the specimens.
Conclusions: These results suggest that suppression of osteoblast differentiation by BIGH3 is one of the mechanisms that enhance osteolytic lesions in RCC bone metastasis, and raise the possibilty that treatments that increase bone formation may improve therapy outcomes.
Copyright © 2018 The Authors. Published by Elsevier Inc. All rights reserved.
Figures






References
-
- Guise TA. The vicious cycle of bone metastases. J Musculoskelet Neuronal Interact. 2002;2:570–572. - PubMed
-
- Mikami S, Katsube K, Oya M, Ishida M, Kosaka T, Mizuno R, Mochizuki S, Ikeda T, Mukai M, Okada Y. Increased RANKL expression is related to tumour migration and metastasis of renal cell carcinomas. J Pathol. 2009;218:530–539. - PubMed
-
- Takahashi N, Udagawa N, Suda T. A new member of tumor necrosis factor ligand family, ODF/OPGL/TRANCE/RANKL, regulates osteoclast differentiation and function. Biochem Biophys Res Commun. 1999;256:449–455. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

