The tissue distribution and significance of B7-H4 in laryngeal carcinoma
- PMID: 29190910
- PMCID: PMC5696176
- DOI: 10.18632/oncotarget.21152
The tissue distribution and significance of B7-H4 in laryngeal carcinoma
Abstract
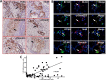
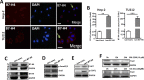
The costimulatory signals CD28 and B7 have been shown to control tumor invasion and metastasis by regulating T cell activation, whereas the distribution characteristics of B7-associated proteins in laryngeal carcinoma (LC) tissue are still unclear. Here, the expression of members of the B7 superfamily, including B7-H1 (PD-L1), B7-DC (PD-L2) and B7-H4, in fifty-two LC samples was determined by immunohistochemistry, and the relationship between B7-H4 and epithelial-mesenchymal transition (EMT)-associated markers was further assessed by immunofluorescence double staining. Furthermore, the human LC cell lines, Hep-2 and TU212 cells, were further transfected to overexpress B7-H4, and cell invasion and metastasis were analyzed. The results showed that B7-H1, B7-DC and B7-H4 were expressed in the tumor cells, and their expression was restricted to the cell membrane and the cytoplasm. The positive rates of these molecules in the tumor tissues were 57.7% (30/52), 32.7% (17/52) and 34.6% (18/52), respectively. Interestingly, double immunofluorescence staining showed that B7-H4 is coexpression with EMT-related markers, including p-Smad2/3, Snail and Vimentin, in carcinoma cells. Moreover, overexpression of B7-H4 in Hep-2 cells promotes the expression of pSmad2/3 and Snail by activating AKT-STAT3 signaling. Transwell and wound-healing assays demonstrated that B7-H4 enhanced both Hep-2 and TU212 cell invasion and metastasis. Our results suggest that B7-H4 transmits feedback signaling to tumor cells and promotes invasion and metastasis by promoting EMT progression. Therefore, blocking B7-H4 signaling might be a novel treatment strategy for LC.
Keywords: B7-H4; EMT; STAT3; invasion and metastasis; laryngeal carcinoma.
Conflict of interest statement
CONFLICTS OF INTEREST The authors declare no financial or commercial conflicts of interest.
Figures




References
-
- Mannelli G, Cecconi L, Gallo O. Laryngeal preneoplastic lesions and cancer: challenging diagnosis. Qualitative literature review and meta-analysis. Crit Rev Oncol Hematol. 2016;106:64–90. - PubMed
-
- Curado MP, Hashibe M. Recent changes in the epidemiology of head and neck cancer. Curr Opin Oncol. 2009;21:194–200. - PubMed
-
- Dedivitis RA, Aires FT, Cernea CR, Brandão LG. Pharyngocutaneous fistula after total laryngectomy: systematic review of risk factors. Head Neck. 2015;37:1691–7. - PubMed
-
- Bellmunt J, Powles T, Vogelzang NJ. A review on the evolution of PD-1/PD-L1 immunotherapy for bladder cancer: the future is now. Cancer Treat Rev. 2017;54:58–67. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

