Viscoelastic properties of human pancreatic tumors and in vitro constructs to mimic mechanical properties
- PMID: 29191507
- PMCID: PMC5797706
- DOI: 10.1016/j.actbio.2017.11.037
Viscoelastic properties of human pancreatic tumors and in vitro constructs to mimic mechanical properties
Abstract
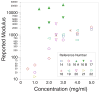
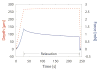
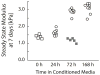
Pancreatic ductal adenocarcinoma (PDAC) is almost universally fatal, in large part due to a protective fibrotic barrier generated by tumor-associated stromal (TAS) cells. This barrier is thought to promote cancer cell survival and confounds attempts to develop effective therapies. We present a 3D in vitro system that replicates the mechanical properties of the PDAC microenvironment, representing an invaluable tool for understanding the biology of the disease. Mesoscale indentation quantified viscoelastic metrics of resected malignant tumors, inflamed chronic pancreatitis regions, and histologically normal tissue. Both pancreatitis (2.15 ± 0.41 kPa, Mean ± SD) and tumors (5.46 ± 3.18 kPa) exhibit higher Steady-State Modulus (SSM) than normal tissue (1.06 ± 0.25 kPa; p < .005). The average viscosity of pancreatitis samples (63.2 ± 26.7 kPa·s) is significantly lower than that of both normal tissue (252 ± 134 kPa·s) and tumors (349 ± 222 kPa·s; p < .005). To mimic this remodeling behavior, PDAC and TAS cells were isolated from human PDAC tumors. Conditioned medium from PDAC cells was used to culture TAS-embedded collagen hydrogels. After 7 days, TAS-embedded gels in control medium reached SSM (1.45 ± 0.12 kPa) near normal pancreas, while gels maintained with conditioned medium achieved higher SSM (3.38 ± 0.146 kPa) consistent with tumors. Taken together, we have demonstrated an in vitro system that recapitulates in vivo stiffening of PDAC tumors. In addition, our quantification of viscoelastic properties suggests that elastography algorithms incorporating viscosity may be able to more accurately distinguish between pancreatic cancer and pancreatitis.
Statement of significance: Understanding tumor-stroma crosstalk in pancreatic ductal adenocarcinoma (PDAC) is challenged by a lack of stroma-mimicking model systems. To design appropriate models, pancreatic tissue must be characterized with a method capable of evaluating in vitro models as well. Our indentation-based characterization tool quantified the distinct viscoelastic signatures of inflamed resections from pancreatitis, tumors from PDAC, and otherwise normal tissue to inform development of mechanically appropriate engineered tissues and scaffolds. We also made progress toward a 3D in vitro system that recapitulates mechanical properties of tumors. Our in vitro model of stromal cells in collagen and complementary characterization system can be used to investigate mechanisms of cancer-stroma crosstalk in PDAC and to propose and test innovative therapies.
Keywords: Cancer associated fibroblasts; Collagen hydrogels; Indentation; Pancreatic ductal adenocarcinoma; Pancreatic stellate cells; Pancreatitis; Tissue mechanics.
Copyright © 2017 Acta Materialia Inc. Published by Elsevier Ltd. All rights reserved.
Conflict of interest statement
The authors have no competing interest to declare.
Figures



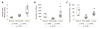

Similar articles
-
Generation of an in vitro 3D PDAC stroma rich spheroid model.Biomaterials. 2016 Nov;108:129-42. doi: 10.1016/j.biomaterials.2016.08.041. Epub 2016 Sep 2. Biomaterials. 2016. PMID: 27627810 Free PMC article.
-
Viscoelastic stiffening of gelatin hydrogels for dynamic culture of pancreatic cancer spheroids.Acta Biomater. 2024 Mar 15;177:203-215. doi: 10.1016/j.actbio.2024.02.010. Epub 2024 Feb 12. Acta Biomater. 2024. PMID: 38354874 Free PMC article.
-
Interleukin 35 Expression Correlates With Microvessel Density in Pancreatic Ductal Adenocarcinoma, Recruits Monocytes, and Promotes Growth and Angiogenesis of Xenograft Tumors in Mice.Gastroenterology. 2018 Feb;154(3):675-688. doi: 10.1053/j.gastro.2017.09.039. Epub 2017 Oct 6. Gastroenterology. 2018. PMID: 28989066
-
Pancreatic stellate cells and pancreas cancer: current perspectives and future strategies.Eur J Cancer. 2014 Oct;50(15):2570-82. doi: 10.1016/j.ejca.2014.06.021. Epub 2014 Aug 1. Eur J Cancer. 2014. PMID: 25091797 Review.
-
Crosstalk between Tumor and Stromal Cells in Pancreatic Ductal Adenocarcinoma.Int J Mol Sci. 2020 Jul 31;21(15):5486. doi: 10.3390/ijms21155486. Int J Mol Sci. 2020. PMID: 32752017 Free PMC article. Review.
Cited by
-
A Tumor Microenvironment Model of Pancreatic Cancer to Elucidate Responses toward Immunotherapy.Adv Healthc Mater. 2023 Jun;12(14):e2201907. doi: 10.1002/adhm.202201907. Epub 2022 Dec 11. Adv Healthc Mater. 2023. PMID: 36417691 Free PMC article.
-
Selection and Optimization of a Bioink Based on PANC-1- Plasma/Alginate/Methylcellulose for Pancreatic Tumour Modelling.Polymers (Basel). 2023 Jul 27;15(15):3196. doi: 10.3390/polym15153196. Polymers (Basel). 2023. PMID: 37571089 Free PMC article.
-
3D approaches to model the tumor microenvironment of pancreatic cancer.Theranostics. 2020 Apr 6;10(11):5074-5089. doi: 10.7150/thno.42441. eCollection 2020. Theranostics. 2020. PMID: 32308769 Free PMC article. Review.
-
Combined effects of matrix stiffness and obesity-associated signaling directs progressive phenotype in PANC-1 pancreatic cancer cells in vitro.bioRxiv [Preprint]. 2024 Jun 14:2024.06.11.598541. doi: 10.1101/2024.06.11.598541. bioRxiv. 2024. PMID: 38915620 Free PMC article. Preprint.
-
Piezo1-induced durotaxis of pancreatic stellate cells depends on TRPC1 and TRPV4 channels.J Cell Sci. 2025 Apr 15;138(8):jcs263846. doi: 10.1242/jcs.263846. Epub 2025 Apr 25. J Cell Sci. 2025. PMID: 40019468 Free PMC article.
References
-
- Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2017. CA Cancer J Clin. 2017;67:7–30. - PubMed
-
- Bhaw-Luximon A, Jhurry D. New avenues for improving pancreatic ductal adenocarcinoma (PDAC) treatment: Selective stroma depletion combined with nano drug delivery. Cancer Lett. 2015;369:266–73. - PubMed
-
- Sugimoto M, Takahashi S, Kojima M, Gotohda N, Kato Y, Kawano S, Ochiai A, Konishi M. What is the nature of pancreatic consistency? Assessment of the elastic modulus of the pancreas and comparison with tactile sensation, histology, and occurrence of postoperative pancreatic fistula after pancreaticoduodenectomy. Surgery. 2014;156:1204–11. - PubMed
-
- Georges PC, Hui JJJ, Gombos Z, McCormick ME, Wang AY, Uemura M, Mick R, Ianmev PA, Furth EE, Wells RG. Increased stiffness of the rat liver precedes matrix deposition: implications for fibrosis. Am J Physiol Gastrointest Liver Physiol. 2007;293:G1147–54. - PubMed
-
- Perepelyuk M, Terajima M, Wang AY, Georges PC, Janmey PA, Yamauchi M, Wells RG. Hepatic stellate cells and portal fibroblasts are the major cellular sources of collagens and lysyl oxidases in normal liver and early after injury. Am J Physiol Gastrointest Liver Physiol. 2013;304:G605–14. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

