Suberanilohydroxamic Acid as a Pharmacological Kruppel-Like Factor 2 Activator That Represses Vascular Inflammation and Atherosclerosis
- PMID: 29191808
- PMCID: PMC5779026
- DOI: 10.1161/JAHA.117.007134
Suberanilohydroxamic Acid as a Pharmacological Kruppel-Like Factor 2 Activator That Represses Vascular Inflammation and Atherosclerosis
Abstract
Background: Kruppel-like factor 2 (KLF2) is an important zinc-finger transcription factor that maintains endothelial homeostasis by its anti-inflammatory, -thrombotic, -oxidative, and -proliferative effects in endothelial cells. In light of the potent vasoprotective effects of KLF2, modulating KLF2 expression or function could give rise to new therapeutic strategies to treat cardiovascular diseases.
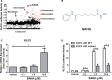
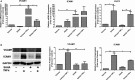
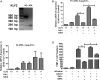
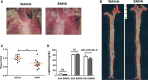
Methods and results: High-throughput drug screening based on KLF2 promoter luciferase reporter assay was performed to screen KLF2 activators. Real-time PCR and western blot were used to detect gene and protein expression. Identified KLF2 activator was orally administered to ApoE-/- mice to evaluate anti-atherosclerotic efficacy. By screening 2400 compounds in the Spectrum library, we identified suberanilohydroxamic (SAHA) acid, also known as vorinostat as a pharmacological KLF2 activator through myocyte enhancer factor 2. We found that SAHA exhibited anti-inflammatory effects and attenuated monocyte adhesion to endothelial cells inflamed with tumor necrosis factor alpha. We further showed that the inhibitory effect of SAHA on endothelial inflammation and ensuing monocyte adhesion was KLF2 dependent using KLF2-deficient mouse lung endothelial cells or KLF2 small interfering RNA- depleted human endothelial cells. Importantly, we observed that oral administration of SAHA reduced diet-induced atherosclerotic lesion development in ApoE-/- mice without significant effect on serum lipid levels.
Conclusions: These results demonstrate that SAHA has KLF2-dependent anti-inflammatory effects in endothelial cells and provide the proof of concept that KLF2 activation could be a promising therapeutic strategy for treating atherosclerosis.
Keywords: SAHA; atherosclerosis; endothelial cell; inflammation.
© 2017 The Authors. Published on behalf of the American Heart Association, Inc., by Wiley.
Figures

Similar articles
-
ERK5/KLF2 activation is involved in the reducing effects of puerarin on monocyte adhesion to endothelial cells and atherosclerotic lesion in apolipoprotein E-deficient mice.Biochim Biophys Acta Mol Basis Dis. 2018 Aug;1864(8):2590-2599. doi: 10.1016/j.bbadis.2018.04.021. Epub 2018 Apr 30. Biochim Biophys Acta Mol Basis Dis. 2018. PMID: 29723698
-
Tannic acid as a plant-derived polyphenol exerts vasoprotection via enhancing KLF2 expression in endothelial cells.Sci Rep. 2017 Jul 27;7(1):6686. doi: 10.1038/s41598-017-06803-x. Sci Rep. 2017. PMID: 28751752 Free PMC article.
-
The anti-inflammatory vasostatin-2 attenuates atherosclerosis in ApoE-/- mice and inhibits monocyte/macrophage recruitment.Thromb Haemost. 2017 Jan 26;117(2):401-414. doi: 10.1160/TH16-06-0475. Epub 2016 Nov 10. Thromb Haemost. 2017. PMID: 27831589
-
Endothelial Krüppel-like factor 2/4: Regulation and function in cardiovascular diseases.Cell Signal. 2025 Jun;130:111699. doi: 10.1016/j.cellsig.2025.111699. Epub 2025 Feb 27. Cell Signal. 2025. PMID: 40023301 Review.
-
Key transcriptional regulators of the vasoprotective effects of shear stress.Hamostaseologie. 2009 Jan;29(1):39-40, 41-3. Hamostaseologie. 2009. PMID: 19151844 Review.
Cited by
-
Transcriptomic analysis of rat prefrontal cortex following chronic stress induced by social isolation - Relevance to psychiatric and neurodevelopmental illness, and implications for treatment.Neurobiol Stress. 2024 Oct 17;33:100679. doi: 10.1016/j.ynstr.2024.100679. eCollection 2024 Nov. Neurobiol Stress. 2024. PMID: 39502833 Free PMC article.
-
Histone Deacetylases (HDACs) and Atherosclerosis: A Mechanistic and Pharmacological Review.Front Cell Dev Biol. 2020 Nov 12;8:581015. doi: 10.3389/fcell.2020.581015. eCollection 2020. Front Cell Dev Biol. 2020. PMID: 33282862 Free PMC article. Review.
-
Role and research progress of histone modification in cardiovascular diseases (Review).Exp Ther Med. 2025 May 13;30(1):132. doi: 10.3892/etm.2025.12882. eCollection 2025 Jul. Exp Ther Med. 2025. PMID: 40421232 Free PMC article. Review.
-
The Involvement of Krüppel-like Factors in Cardiovascular Diseases.Life (Basel). 2023 Feb 2;13(2):420. doi: 10.3390/life13020420. Life (Basel). 2023. PMID: 36836777 Free PMC article. Review.
-
Epigenetic Signatures in Arterial Hypertension: Focus on the Microvasculature.Int J Mol Sci. 2023 Mar 2;24(5):4854. doi: 10.3390/ijms24054854. Int J Mol Sci. 2023. PMID: 36902291 Free PMC article. Review.
References
-
- Novodvorsky P, Chico TJ. The role of the transcription factor KLF2 in vascular development and disease. Prog Mol Biol Transl Sci. 2014;124:155–188. - PubMed
-
- Gimbrone MA Jr. Endothelial dysfunction, hemodynamic forces, and atherosclerosis. Thromb Haemost. 1999;82:722–726. - PubMed
-
- Marrone G, Russo L, Rosado E, Hide D, Garcia‐Cardena G, Garcia‐Pagan JC, Bosch J, Gracia‐Sancho J. The transcription factor KLF2 mediates hepatic endothelial protection and paracrine endothelial‐stellate cell deactivation induced by statins. J Hepatol. 2013;58:98–103. - PubMed
-
- Atkins GB, Jain MK. Role of Kruppel‐like transcription factors in endothelial biology. Circ Res. 2007;100:1686–1695. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

