Hydrogen-deuterium exchange reveals long-range dynamical allostery in soybean lipoxygenase
- PMID: 29191828
- PMCID: PMC5787793
- DOI: 10.1074/jbc.M117.817197
Hydrogen-deuterium exchange reveals long-range dynamical allostery in soybean lipoxygenase
Abstract
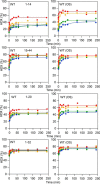
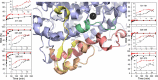
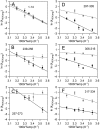
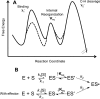
In lipoxygenases, the topologically conserved C-terminal domain catalyzes the oxidation of polyunsaturated fatty acids, generating an assortment of biologically relevant signaling mediators. Plant and animal lipoxygenases also contain a 100-150-amino acid N-terminal C2-like domain that has been implicated in interactions with isolated fatty acids and at the phospholipid bilayer. These interactions may lead to increased substrate availability and contribute to the regulation of active-site catalysis. Because of a lack of structural information, a molecular understanding of this lipid-protein interaction remains unresolved. Herein, we employed hydrogen-deuterium exchange MS (HDXMS) to spatially resolve changes in protein conformation upon interaction of soybean lipoxygenase with a fatty acid surrogate, oleyl sulfate (OS), previously shown to act at a site separate from the substrate-binding site. Specific, OS-induced conformational changes are detected both at the N-terminal domain and within the substrate portal nearly 30 Å away. Combining previously measured kinetic properties in the presence of OS with its impact on the Kd for linoleic acid substrate binding, we conclude that OS binding brings about an increase in rate constants for both the ingress and egress of substrate. We discuss the role of OS-induced changes in protein flexibility in the context of changes in the mechanism of substrate acquisition.
Keywords: SLO; allosteric regulation; allostery; enzyme inhibitor; fatty acid; hydrogen exchange mass spectrometry; hydrogen-deuterium exchange (HDX); inhibition mechanism; lipoxygenase.
© 2018 by The American Society for Biochemistry and Molecular Biology, Inc.
Conflict of interest statement
The authors declare that they have no conflicts of interest with the contents of this article
Figures





References
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

