Osteoblasts remotely supply lung tumors with cancer-promoting SiglecFhigh neutrophils
- PMID: 29191879
- PMCID: PMC6343476
- DOI: 10.1126/science.aal5081
Osteoblasts remotely supply lung tumors with cancer-promoting SiglecFhigh neutrophils
Abstract
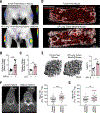
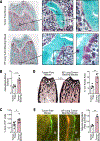
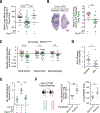
Bone marrow-derived myeloid cells can accumulate within tumors and foster cancer outgrowth. Local immune-neoplastic interactions have been intensively investigated, but the contribution of the systemic host environment to tumor growth remains poorly understood. Here, we show in mice and cancer patients (n = 70) that lung adenocarcinomas increase bone stromal activity in the absence of bone metastasis. Animal studies reveal that the cancer-induced bone phenotype involves bone-resident osteocalcin-expressing (Ocn+) osteoblastic cells. These cells promote cancer by remotely supplying a distinct subset of tumor-infiltrating SiglecFhigh neutrophils, which exhibit cancer-promoting properties. Experimentally reducing Ocn+ cell numbers suppresses the neutrophil response and lung tumor outgrowth. These observations posit osteoblasts as remote regulators of lung cancer and identify SiglecFhigh neutrophils as myeloid cell effectors of the osteoblast-driven protumoral response.
Copyright © 2017 The Authors, some rights reserved; exclusive licensee American Association for the Advancement of Science. No claim to original U.S. Government Works.
Figures
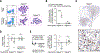

Comment in
-
Bone voyage-Osteoblasts remotely control tumors.Science. 2017 Dec 1;358(6367):1127-1128. doi: 10.1126/science.aar2640. Science. 2017. PMID: 29191891 No abstract available.
-
A Long-Distance Relay-tionship between Tumor and Bone.Immunity. 2018 Jan 16;48(1):13-16. doi: 10.1016/j.immuni.2017.12.011. Immunity. 2018. PMID: 29343434
-
Bone Talk: Activated Osteoblasts Promote Lung Cancer Growth.Trends Mol Med. 2018 Mar;24(3):237-239. doi: 10.1016/j.molmed.2018.01.007. Epub 2018 Feb 3. Trends Mol Med. 2018. PMID: 29402707
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

