EMA Review of Panobinostat (Farydak) for the Treatment of Adult Patients with Relapsed and/or Refractory Multiple Myeloma
- PMID: 29192015
- PMCID: PMC5947444
- DOI: 10.1634/theoncologist.2017-0301
EMA Review of Panobinostat (Farydak) for the Treatment of Adult Patients with Relapsed and/or Refractory Multiple Myeloma
Erratum in
-
EMA Review of Panobinostat (Farydak) for the Treatment of Adult Patients with Relapsed and/or Refractory Multiple Myeloma.Oncologist. 2018 Jul;23(7):870. doi: 10.1634/theoncologist.2017-0301erratum. Oncologist. 2018. PMID: 30037941 Free PMC article. No abstract available.
Abstract
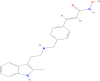
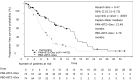
On August 28, 2015, a marketing authorization valid through the European Union was issued for panobinostat, in combination with bortezomib and dexamethasone, for the treatment of adult patients with relapsed and/or refractory multiple myeloma who have received at least two prior regimens including bortezomib and an immunomodulatory agent (IMiD).Panobinostat is an orally available histone deacetylase (HDAC) inhibitor that inhibits the enzymatic activity of HDAC proteins at nanomolar concentrations. HDAC proteins catalyze the removal of acetyl groups from the lysine residues of histones and some nonhistone proteins. Inhibition of HDAC activity results in increased acetylation of histone proteins, an epigenetic alteration that results in a relaxing of chromatin, leading to transcriptional activation. The recommended starting dose of panobinostat is 20 mg, taken orally in a cyclical manner for up to 48 weeks.The use of panobinostat in combination with bortezomib and dexamethasone was studied in a randomized, double-blind, placebo-controlled, multicenter phase III study (PANORAMA I) in 768 patients with relapsed or relapsed and refractory multiple myeloma who had received one to three prior lines of therapies. In the subgroup of patients who have received at least two prior regimens including bortezomib and an IMiD, there was a difference of 7.8 months in the progression-free survival in favor of the experimental arm (12.5 months for panobinostat + bortezomib + dexamethasone vs. 4.7 months for placebo + bortezomib + dexamethasone; hazard ratio = 0.47, 95% confidence interal 0.31-0.72; log-rank p value = .0003). The incidence of grade 3-4 adverse events suspected to be related to study drug was 76.9% vs. 51.2%, for the panobinostat and the placebo group, respectively. The most common side effects (grade 3-4) associated with panobinostat included diarrhea (18.9%), fatigue (14.7%), nausea (4.5%), vomiting (5.5%), thrombocytopenia (43.6%), anemia (7.9%), neutropenia (16.5%) and lymphopenia (8.1%).This article summarizes the scientific review of the application leading to regulatory approval in the European Union. The full scientific assessment report and product information, including the Summary of Product Characteristics, are available on the European Medicines Agency website (http://www.ema.europa.eu/ema/index.jsp?curl=pages/includes/medicines/medicines_landing_page.jsp&mid=).
Implications for practice: Farydak was approved in the European Union in combination with bortezomib and dexamethasone, for the treatment of adult patients with relapsed and/or refractory multiple myeloma who have received at least two prior regimens including bortezomib and an immunomodulatory agent (IMiD). The addition of panobinostat to bortezomib and dexamethasone resulted in a clinically meaningful and statistically significant improvement of progression-free survival compared with bortezomib and dexamethasone, and an additional therapeutic option with a new mechanism of action was considered valuable. Although the toxicity associated with panobinostat combination was significant, at the time of the marketing authorization of panobinostat, it was considered that it was acceptable and that it should be left to the clinician and the patient to decide whether the panobinostat combination is the preferred treatment option or not.
Keywords: European Medicines Agency; Multiple myeloma; Panobinostat.
© AlphaMed Press 2017.
Conflict of interest statement
Disclosures of potential conflicts of interest may be found at the end of this article.
Figures
References
-
- Kyle RA, Rajkumar SV. Multiple myeloma. N Engl J Med 2004;351:1860–1873. - PubMed
-
- Ferlay J, Steliarova‐Foucher E, Lortet‐Tieulent J et al. Cancer incidence and mortality patterns in Europe: Estimates for 40 countries in 2012. Eur J Cancer 2013;49:1374–1403. - PubMed
-
- Kyle RA, Gertz MA, Witzig TE et al. Review of 1027 patients with newly diagnosed multiple myeloma. Mayo Clin Proc 2003;78:21–33. - PubMed
-
- Bladé J, Samson D, Reece D et al. Criteria for evaluating disease response and progression in patients with multiple myeloma treated by high‐dose therapy and haemopoietic stem cell transplantation. Myeloma Subcommittee of the EBMT. European Group for Blood and Marrow Transplant. Br J Haematol 1998;102:1115–1123. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

