Fyn-binding protein ADAP supports actin organization in podocytes
- PMID: 29192064
- PMCID: PMC5727265
- DOI: 10.14814/phy2.13483
Fyn-binding protein ADAP supports actin organization in podocytes
Abstract
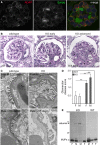
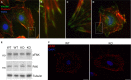
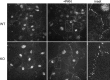
The renal podocyte is central to the filtration function of the kidney that is dependent on maintaining both highly organized, branched cell structures forming foot processes, and a unique cell-cell junction, the slit diaphragm. Our recent studies investigating the developmental formation of the slit diaphragm identified a novel claudin family tetraspannin, TM4SF10, which is a binding partner for ADAP (also known as Fyn binding protein Fyb). To investigate the role of ADAP in podocyte function in relation to Fyn and TM4SF10, we examined ADAP knockout (KO) mice and podocytes. ADAP KO mice developed glomerular pathology that began as hyalinosis and progressed to glomerulosclerosis, with aged male animals developing low levels of albuminuria. Podocyte cell lines established from the KO mice had slower attachment kinetics compared to wild-type cells, although this did not affect the total number of attached cells nor the ability to form focal contacts. After attachment, the ADAP KO cells did not attain typical podocyte morphology, lacking the elaborate cell protrusions typical of wild-type podocytes, with the actin cytoskeleton forming circumferential stress fibers. The absence of ADAP did not alter Fyn levels nor were there differences between KO and wild-type podocytes in the reduction of Fyn activating phosphorylation events with puromycin aminonucleoside treatment. In the setting of endogenous TM4SF10 overexpression, the absence of ADAP altered the formation of cell-cell contacts containing TM4SF10. These studies suggest ADAP does not alter Fyn activity in podocytes, but appears to mediate downstream effects of Fyn controlled by TM4SF10 involving actin cytoskeleton organization.
Keywords: Cell junction; podocyte; proteinuria.
© 2017 The Authors. Physiological Reports published by Wiley Periodicals, Inc. on behalf of The Physiological Society and the American Physiological Society.
Figures



References
-
- Bruggeman, L. A. , Martinka S., and Simske J. S.. 2007. Expression of TM4SF10, a Claudin/EMP/PMP22 family cell junction protein, during mouse kidney development and podocyte differentiation. Dev. Dyn. 236:596–605. - PubMed
-
- Coppolino, M. G. , Krause M., Hagendorff P., Monner D. A., Trimble W., Grinstein S., et al. 2001. Evidence for a molecular complex consisting of Fyb/SLAP, SLP‐76, Nck, VASP and WASP that links the actin cytoskeleton to Fcgamma receptor signalling during phagocytosis. J. Cell Sci. 114:4307–4318. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

