A Copper-Catalyzed Tandem Cyclization Reaction of Aminoalkynes with Alkynes for the Construction of Tetrahydropyrrolo[1,2-a]quinolines Scaffold
- PMID: 29192158
- PMCID: PMC5709353
- DOI: 10.1038/s41598-017-16887-0
A Copper-Catalyzed Tandem Cyclization Reaction of Aminoalkynes with Alkynes for the Construction of Tetrahydropyrrolo[1,2-a]quinolines Scaffold
Abstract
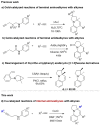
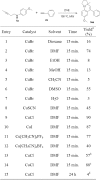
A synthetic method for diversely substituted tetrahydropyrrolo[1,2-a]quinolines was developed via CuCl-catalyzed cascade transformation of internal aminoalkynes with alkynes under microwave- irradiation.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures







References
LinkOut - more resources
Full Text Sources
Other Literature Sources

