Fruit fracture biomechanics and the release of Lepidium didymum pericarp-imposed mechanical dormancy by fungi
- PMID: 29192192
- PMCID: PMC5709442
- DOI: 10.1038/s41467-017-02051-9
Fruit fracture biomechanics and the release of Lepidium didymum pericarp-imposed mechanical dormancy by fungi
Abstract
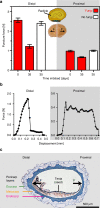
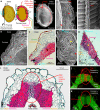
The biomechanical and ecophysiological properties of plant seed/fruit structures are fundamental to survival in distinct environments. Dispersal of fruits with hard pericarps (fruit coats) encasing seeds has evolved many times independently within taxa that have seed dispersal as their default strategy. The mechanisms by which the constraint of a hard pericarp determines germination timing in response to the environment are currently unknown. Here, we show that the hard pericarp of Lepidium didymum controls germination solely by a biomechanical mechanism. Mechanical dormancy is conferred by preventing full phase-II water uptake of the encased non-dormant seed. The lignified endocarp has biomechanically and morphologically distinct regions that serve as predetermined breaking zones. This pericarp-imposed mechanical dormancy is released by the activity of common fungi, which weaken these zones by degrading non-lignified pericarp cells. We propose that the hard pericarp with this biomechanical mechanism contributed to the global distribution of this species in distinct environments.
Conflict of interest statement
The authors declare no competing financial interests.
Figures




References
-
- Steinbrecher T, Leubner-Metzger G. The biomechanics of seed germination. J. Exp. Bot. 2017;68:765–783. - PubMed
-
- Baskin, C. C. & Baskin, J. M. Seeds—Ecology, Biogeography, and Evolution of Dormancy and Germination (Academic Press, San Diego, 2014).
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

