Tunneling nanotubes (TNT) mediate long-range gap junctional communication: Implications for HIV cell to cell spread
- PMID: 29192225
- PMCID: PMC5709493
- DOI: 10.1038/s41598-017-16600-1
Tunneling nanotubes (TNT) mediate long-range gap junctional communication: Implications for HIV cell to cell spread
Abstract
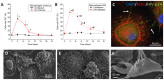
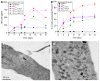
Cell-to-cell communication is essen for the development of multicellular systems and is coordinated by soluble factors, exosomes, gap junction (GJ) channels, and the recently described tunneling nanotubes (TNTs). We and others have demonstrated that TNT-like structures are mostly present during pathogenic conditions, including HIV infection. However, the nature, function, and communication properties of TNTs are still poorly understood. In this manuscript, we demonstrate that TNTs induced by HIV infection have functional GJs at the ends of their membrane extensions and that TNTs mediate long-range GJ communication during HIV infection. Blocking or reducing GJ communication during HIV infection resulted in aberrant TNT cell-to-cell contact, compromising HIV spread and replication. Thus, TNTs and associated GJs are required for the efficient cell-to-cell communication and viral spread. Our data indicate that targeting TNTs/GJs may provide new therapeutic opportunities for the treatment of HIV.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


References
-
- Abounit S, Delage E, Zurzolo C. Identification and Characterization of Tunneling Nanotubes for Intercellular Trafficking. Curr Protoc Cell Biol. 2015;67:12 10 11–21. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

