Access to a main alphaherpesvirus receptor, located basolaterally in the respiratory epithelium, is masked by intercellular junctions
- PMID: 29192251
- PMCID: PMC5709510
- DOI: 10.1038/s41598-017-16804-5
Access to a main alphaherpesvirus receptor, located basolaterally in the respiratory epithelium, is masked by intercellular junctions
Abstract
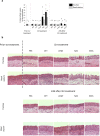
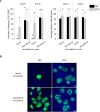
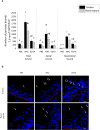
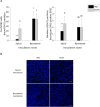
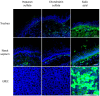
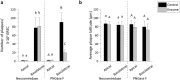
The respiratory epithelium of humans and animals is frequently exposed to alphaherpesviruses, originating from either external exposure or reactivation from latency. To date, the polarity of alphaherpesvirus infection in the respiratory epithelium and the role of respiratory epithelial integrity herein has not been studied. Equine herpesvirus type 1 (EHV1), a well-known member of the alphaherpesvirus family, was used to infect equine respiratory mucosal explants and primary equine respiratory epithelial cells (EREC), grown at the air-liquid interface. EHV1 binding to and infection of mucosal explants was greatly enhanced upon destruction of the respiratory epithelium integrity with EGTA or N-acetylcysteine. EHV1 preferentially bound to and entered EREC at basolateral cell surfaces. Restriction of infection via apical inoculation was overcome by disruption of intercellular junctions. Finally, basolateral but not apical EHV1 infection of EREC was dependent on cellular N-linked glycans. Overall, our findings demonstrate that integrity of the respiratory epithelium is crucial in the host's innate defence against primary alphaherpesvirus infections. In addition, by targeting a basolaterally located receptor in the respiratory epithelium, alphaherpesviruses have generated a strategy to efficiently escape from host defence mechanisms during reactivation from latency.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

Similar articles
-
An Alphaherpesvirus Exploits Antimicrobial β-Defensins To Initiate Respiratory Tract Infection.J Virol. 2020 Mar 31;94(8):e01676-19. doi: 10.1128/JVI.01676-19. Print 2020 Mar 31. J Virol. 2020. PMID: 31996426 Free PMC article.
-
Deoxynivalenol, but not fumonisin B1, aflatoxin B1 or diesel exhaust particles disrupt integrity of the horse's respiratory epithelium and predispose it for equine herpesvirus type 1 infection.Vet Microbiol. 2019 Jul;234:17-24. doi: 10.1016/j.vetmic.2019.05.009. Epub 2019 May 9. Vet Microbiol. 2019. PMID: 31213268
-
Equine Herpesvirus 1 Bridles T Lymphocytes To Reach Its Target Organs.J Virol. 2019 Mar 21;93(7):e02098-18. doi: 10.1128/JVI.02098-18. Print 2019 Apr 1. J Virol. 2019. PMID: 30651370 Free PMC article.
-
Antiviral agents against equid alphaherpesviruses: Current status and perspectives.Vet J. 2016 Jan;207:38-44. doi: 10.1016/j.tvjl.2015.06.010. Epub 2015 Jun 12. Vet J. 2016. PMID: 26654843 Review.
-
The equine immune response to equine herpesvirus-1: the virus and its vaccines.Vet Immunol Immunopathol. 2006 May 15;111(1-2):15-30. doi: 10.1016/j.vetimm.2006.01.005. Epub 2006 Feb 14. Vet Immunol Immunopathol. 2006. PMID: 16476492 Review.
Cited by
-
Update on human herpesvirus 7 pathogenesis and clinical aspects as a roadmap for future research.J Virol. 2024 Jun 13;98(6):e0043724. doi: 10.1128/jvi.00437-24. Epub 2024 May 8. J Virol. 2024. PMID: 38717112 Free PMC article. Review.
-
Pseudorabies virus infection increases the permeability of the mammalian respiratory barrier to facilitate Pasteurella multocida infection.mSphere. 2024 Aug 28;9(8):e0029724. doi: 10.1128/msphere.00297-24. Epub 2024 Jul 23. mSphere. 2024. PMID: 39041808 Free PMC article.
-
Pollens destroy respiratory epithelial cell anchors and drive alphaherpesvirus infection.Sci Rep. 2019 Mar 18;9(1):4787. doi: 10.1038/s41598-019-41305-y. Sci Rep. 2019. PMID: 30886217 Free PMC article.
-
Gammaherpesvirus BoHV-4 infects bovine respiratory epithelial cells mainly at the basolateral side.Vet Res. 2019 Feb 8;50(1):11. doi: 10.1186/s13567-019-0629-z. Vet Res. 2019. PMID: 30736853 Free PMC article.
-
Genomic analysis and replication kinetics of the closely related EHV-1 neuropathogenic 21P40 and abortigenic 97P70 strains.Vet Res. 2025 Jan 13;56(1):12. doi: 10.1186/s13567-024-01434-3. Vet Res. 2025. PMID: 39806433 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

