Cerebral microcirculatory alterations and the no-reflow phenomenon in vivo after experimental pediatric cardiac arrest
- PMID: 29192562
- PMCID: PMC6501505
- DOI: 10.1177/0271678X17744717
Cerebral microcirculatory alterations and the no-reflow phenomenon in vivo after experimental pediatric cardiac arrest
Abstract
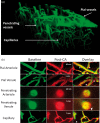
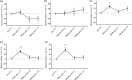
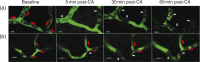
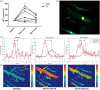
Decreased cerebral blood flow (CBF) after cardiac arrest (CA) contributes to secondary ischemic injury in infants and children. We previously reported cortical hypoperfusion with tissue hypoxia early in a pediatric rat model of asphyxial CA. In order to identify specific alterations as potential therapeutic targets to improve cortical hypoperfusion post-CA, we characterize the CBF alterations at the cortical microvascular level in vivo using multiphoton microscopy. We hypothesize that microvascular constriction and disturbances of capillary red blood cell (RBC) flow contribute to cortical hypoperfusion post-CA. After resuscitation from 9 min asphyxial CA, transient dilation of capillaries and venules at 5 min was followed by pial arteriolar constriction at 30 and 60 min (19.6 ± 1.3, 19.3 ± 1.2 µm at 30, 60 min vs. 22.0 ± 1.2 µm at baseline, p < 0.05). At the capillary level, microcirculatory disturbances were highly heterogeneous, with RBC stasis observed in 25.4% of capillaries at 30 min post-CA. Overall, the capillary plasma mean transit time was increased post-CA by 139.7 ± 51.5%, p < 0.05. In conclusion, pial arteriolar constriction, the no-reflow phenomenon and increased plasma transit time were observed post-CA. Our results detail the microvascular disturbances in a pediatric asphyxial CA model and provide a powerful platform for assessing specific vascular-targeted therapies.
Keywords: Cardiac arrest; cerebral blood flow; mean transit time; microcirculation; no-reflow phenomenon; pediatric.
Figures

References
-
- Rossano JW, Naim MY, Nadkarni VM, et al. Epidemiology of pediatric cardiac arrest. In: Cruz EM, Ivy D, Jaggers J (eds). Pediatric and congenital cardiology, cardiac surgery and intensive care, London: Springer, 2014, pp. 1275–1287.
-
- Nadkarni VM, Larkin GL, Peberdy MA, et al. First documented rhythm and clinical outcome from in-hospital cardiac arrest among children and adults. JAMA 2006; 295: 50–57. - PubMed
-
- Leonov Y, Sterz F, Safar P, et al. Hypertension with hemodilution prevents multifocal cerebral hypoperfusion after cardiac arrest in dogs. Stroke 1992; 23: 45–53. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

