The histopathology of Erdheim-Chester disease: a comprehensive review of a molecularly characterized cohort
- PMID: 29192649
- PMCID: PMC6718953
- DOI: 10.1038/modpathol.2017.160
The histopathology of Erdheim-Chester disease: a comprehensive review of a molecularly characterized cohort
Abstract
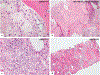
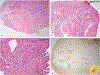
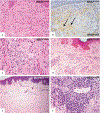
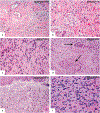
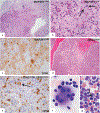
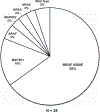
Erdheim-Chester disease is a rare, non-Langerhans cell histiocytosis histologically characterized by multi-systemic proliferation of mature histiocytes in a background of inflammatory stroma. The disease can involve virtually any organ system; most commonly the bones, skin, retroperitoneum, heart, orbit, lung, and brain are affected. Although a histiocytic proliferation is the histological hallmark of the disease, a wide range of morphological appearances have been described as part of case studies or small series. A comprehensive review of histopathological features in clinically and molecularly defined Erdheim-Chester disease has yet to be characterized. To address this issue and help guide clinical practice, we comprehensively analyzed the pathological spectrum of Erdheim-Chester disease in a clinically and molecularly defined cohort. We reviewed 73 biopsies from 42 patients showing involvement by histiocytosis from a variety of organ systems, including bone (16), retroperitoneum (11), skin (19), orbit (6), brain (5), lung (6), cardiac structures (2), epidural soft tissue (3), oral cavity (2), subcutaneous soft tissue (2), and testis (2). In eight patients, one or more bone marrow biopsies were performed due to clinical indication and an accompanying myeloid neoplasm was detected in six of them. Thirty-eight cases were investigated for genetic abnormalities. Somatic mutations involving BRAF (25/38), MAP2K1 (6/38), ARAF (2/38), MAP2K2 (1/38), KRAS (1/38), and NRAS (1/38) genes were detected. One of the cases with a MAP2K1 mutation also harbored a PIK3CA mutation. We have observed marked heterogeneity in histology and immunophenotype, identified site-specific features, overlap with other histiocytic and myeloid disorders and potential diagnostic pitfalls. We hope that broadening the spectrum of recognized pathologic manifestations of Erdheim-Chester disease will help practicing clinicians and pathologists to diagnose Erdheim-Chester disease early in the disease course and manage these patients effectively.
Figures


References
-
- Cavalli G, Guglielmi B, Berti A, et al. The multifaceted clinical presentations and manifestations of Erdheim–Chester disease: comprehensive review of the literature and of 10 new cases. Ann Rheum Dis 2013;72:1691–1695. - PubMed
-
- Arnaud L, Gorochov G, Charlotte F, et al. Systemic perturbation of cytokine and chemokine networks in Erdheim–Chester disease: a single-center series of 37 patients. Blood 2011;117:2783–2790. - PubMed
-
- Haroche J, Charlotte F, Arnaud L, et al. High prevalence of BRAF V600E mutations in Erdheim–Chester disease but not in other non-Langerhans cell histiocytoses. Blood 2012;120:2700–2703. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

