Experimental study of delivery of humidified-warm carbon dioxide during open abdominal surgery
- PMID: 29193022
- PMCID: PMC5901019
- DOI: 10.1002/bjs.10685
Experimental study of delivery of humidified-warm carbon dioxide during open abdominal surgery
Abstract
Background: The aim of this study was to monitor the effect of humidified-warm carbon dioxide (HWCO2 ) delivered into the open abdomen of mice, simulating laparotomy.
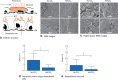
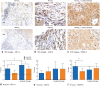
Methods: Mice were anaesthetized, ventilated and subjected to an abdominal incision followed by wound retraction. In the experimental group, a diffuser device was used to deliver HWCO2 ; the control group was exposed to passive air flow. In each group of mice, surgical damage was produced on one side of the peritoneal wall. Vital signs and core temperature were monitored throughout the 1-h procedure. The peritoneum was closed and mice were allowed to recover for 24 h or 10 days. Tumour cells were delivered into half of the mice in each cohort. Tissue was then examined using scanning electron microscopy and immunohistochemistry.
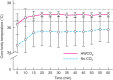
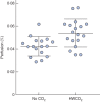
Results: Passive air flow generated ultrastructural damage including mesothelial cell bulging/retraction and loss of microvilli, as assessed at 24 h. Evidence of surgical damage was still measurable on day 10. HWCO2 maintained normothermia, whereas open surgery alone led to hypothermia. The degree of tissue damage was significantly reduced by HWCO2 compared with that in controls. Peritoneal expression of hypoxia inducible factor 1α and vascular endothelial growth factor A was lowered by HWCO2 . These effects were also evident at the surgical damage sites, where protection from tissue trauma extended to 10 days. HWCO2 did not reduce tumorigenesis in surgically damaged sites compared with passive air flow.
Conclusion: HWCO2 diffusion into the abdomen in the context of open surgery afforded tissue protection and accelerated tissue repair in mice, while preserving normothermia. Surgical relevance Damage to the peritoneum always occurs during open abdominal surgery, by exposure to desiccating air and by mechanical trauma/damage owing to the surgical intervention. Previous experimental studies showed that humidified-warm carbon dioxide (HWCO2 ) reduced peritoneal damage during laparoscopic insufflation. Additionally, this intervention decreased experimental peritoneal carcinomatosis compared with the use of conventional dry-cold carbon dioxide. In the present experimental study, the simple delivery of HWCO2 into the open abdomen reduced the amount of cellular damage and inflammation, and accelerated tissue repair. Sites of surgical intervention serve as ideal locations for cancer cell adhesion and subsequent tumour formation, but this was not changed measurably by the delivery of HWCO2 .
© 2017 The Authors. BJS published by John Wiley & Sons Ltd on behalf of BJS Society Ltd.
Figures


Similar articles
-
Peritoneal Tumorigenesis and Inflammation are Ameliorated by Humidified-Warm Carbon Dioxide Insufflation in the Mouse.Ann Surg Oncol. 2015 Dec;22 Suppl 3(Suppl 3):S1540-7. doi: 10.1245/s10434-015-4508-1. Epub 2015 Mar 21. Ann Surg Oncol. 2015. PMID: 25794828 Free PMC article.
-
Heated and humidified CO2 prevents hypothermia, peritoneal injury, and intra-abdominal adhesions during prolonged laparoscopic insufflations.J Surg Res. 2009 Jan;151(1):40-7. doi: 10.1016/j.jss.2008.03.039. Epub 2008 Apr 23. J Surg Res. 2009. PMID: 18639246
-
Laparotomy causes loss of peritoneal mesothelium prevented by humidified CO2 insufflation in rats.J Surg Res. 2017 Dec;220:300-310. doi: 10.1016/j.jss.2017.06.057. Epub 2017 Aug 12. J Surg Res. 2017. PMID: 29180196
-
Humidification during laparoscopic surgery: overview of the clinical benefits of using humidified gas during laparoscopic surgery.Arch Gynecol Obstet. 2015 Nov;292(5):955-71. doi: 10.1007/s00404-015-3717-y. Epub 2015 Apr 25. Arch Gynecol Obstet. 2015. PMID: 25911545 Free PMC article. Review.
-
Warmed and humidified carbon dioxide for abdominal laparoscopic surgery: meta-analysis of the current literature.Surg Endosc. 2017 Jan;31(1):1-12. doi: 10.1007/s00464-016-4866-1. Epub 2016 Mar 22. Surg Endosc. 2017. PMID: 27005288 Review.
Cited by
-
Post-surgical adhesions are triggered by calcium-dependent membrane bridges between mesothelial surfaces.Nat Commun. 2020 Jun 17;11(1):3068. doi: 10.1038/s41467-020-16893-3. Nat Commun. 2020. PMID: 32555155 Free PMC article.
-
Clinical effects of warmed humidified carbon dioxide insufflation in infants undergoing major laparoscopic surgery.Medicine (Baltimore). 2019 Jul;98(27):e16151. doi: 10.1097/MD.0000000000016151. Medicine (Baltimore). 2019. PMID: 31277116 Free PMC article. Clinical Trial.
-
Randomized clinical trial of the effect of intraoperative humidified carbon dioxide insufflation in open laparotomy for colorectal resection.BJS Open. 2020 Feb;4(1):45-58. doi: 10.1002/bjs5.50227. Epub 2019 Nov 17. BJS Open. 2020. PMID: 32011809 Free PMC article. Clinical Trial.
-
Modes of carbon dioxide delivery during laparoscopy generate distinct differences in peritoneal damage and hypoxia in a porcine model.Surg Endosc. 2020 Oct;34(10):4395-4402. doi: 10.1007/s00464-019-07213-y. Epub 2019 Oct 17. Surg Endosc. 2020. PMID: 31624943
-
Modeling open surgery in mice to explore peritoneal damage, carbon dioxide humidification and desmoidogenesis.Pleura Peritoneum. 2019 Nov 2;4(4):20190023. doi: 10.1515/pp-2019-0023. eCollection 2019 Dec 1. Pleura Peritoneum. 2019. PMID: 31799374 Free PMC article.
References
-
- Dharan S, Pittet D. Environmental controls in operating theatres. J Hosp Infect 2002; 51: 79–84. - PubMed
-
- Persson M, van der Linden J. Intraoperative field flooding with warm humidified CO2 may help to prevent adhesion formation after open surgery. Med Hypotheses 2009; 73: 521–523. - PubMed
-
- Kaudasch G, Schempp P, Skierski P, Turner E. [The effect of convection warming during abdominal surgery on the early postoperative heat balance.] Anaesthesist 1996; 45: 1075–1081. - PubMed
-
- Lindic J, Psenicnik M, Bren A, Gucek A, Ferluga D, Kveder R. The morphology of parietal peritoneum: a scanning electron micrograph study. Adv Perit Dial 1993; 9: 36–38. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

