Stereological assessment of the blood-air barrier and the surfactant system after mesenchymal stem cell pretreatment in a porcine non-heart-beating donor model for lung transplantation
- PMID: 29193065
- PMCID: PMC5770329
- DOI: 10.1111/joa.12747
Stereological assessment of the blood-air barrier and the surfactant system after mesenchymal stem cell pretreatment in a porcine non-heart-beating donor model for lung transplantation
Abstract
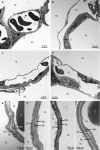
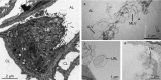
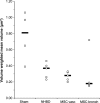
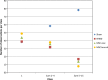
More frequent utilization of non-heart-beating donor (NHBD) organs for lung transplantation has the potential to relieve the shortage of donor organs. In particular with respect to uncontrolled NHBD, concerns exist regarding the risk of ischaemia/reperfusion (IR) injury-related graft damage or dysfunction. Due to their immunomodulating and tissue-remodelling properties, bone-marrow-derived mesenchymal stem cells (MSCs) have been suspected of playing a beneficial role regarding short- and long-term survival and function of the allograft. Thus, MSC administration might represent a promising pretreatment strategy for NHBD organs. To study the initial effects of warm ischaemia and MSC application, a large animal lung transplantation model was generated, and the structural organ composition of the transplanted lungs was analysed stereologically with particular respect to the blood-gas barrier and the surfactant system. In this study, porcine lungs (n = 5/group) were analysed. Group 1 was the sham-operated control group. In pigs of groups 2-4, cardiac arrest was induced, followed by a period of 3 h of ventilated ischaemia at room temperature. In groups 3 and 4, 50 × 106 MSCs were administered intravascularly via the pulmonary artery and endobronchially, respectively, during the last 10 min of ischaemia. The left lungs were transplanted, followed by a reperfusion period of 4 h. Then, lungs were perfusion-fixed and processed for light and electron microscopy. Samples were analysed stereologically for IR injury-related structural parameters, including volume densities and absolute volumes of parenchyma components, alveolar septum components, intra-alveolar oedema, and the intracellular and intra-alveolar surfactant pool. Additionally, the volume-weighted mean volume of lamellar bodies (lbs) and their profile size distribution were determined. Three hours of ventilated warm ischaemia was tolerated without eliciting histological or ultrastructural signs of IR injury, as revealed by qualitative and quantitative assessment. However, warm ischaemia influenced the surfactant system. The volume-weighted mean volume of lbs was reduced significantly (P = 0.024) in groups subjected to ischaemia (group medians of groups 2-4: 0.180-0.373 μm³) compared with the sham control group (median 0.814 μm³). This was due to a lower number of large lb profiles (size classes 5-15). In contrast, the intra-alveolar surfactant system was not altered significantly. No significant differences were encountered comparing ischaemia alone (group 2) or ischaemia plus application of MSCs (groups 3 and 4) in this short-term model.
Keywords: lung transplantation; mesenchymal stem cells; non-heart-beating donor; stereology; surfactant system.
© 2017 Anatomical Society.
Figures

Similar articles
-
Effects of exogenous surfactant on the non-heart-beating donor lung graft in experimental lung transplantation - a stereological study.J Anat. 2014 May;224(5):594-602. doi: 10.1111/joa.12167. Epub 2014 Feb 14. J Anat. 2014. PMID: 24527871 Free PMC article.
-
Mesenchymal stem cell pretreatment of non-heart-beating-donors in experimental lung transplantation.J Cardiothorac Surg. 2014 Sep 2;9:151. doi: 10.1186/s13019-014-0151-3. J Cardiothorac Surg. 2014. PMID: 25179441 Free PMC article.
-
Continuous infusion of nitroglycerin improves pulmonary graft function of non-heart-beating donor lungs.Transplantation. 2004 Jun 27;77(12):1803-8. doi: 10.1097/01.tp.0000131155.81609.37. Transplantation. 2004. PMID: 15223895
-
The effects of ischaemic conditioning on lung ischaemia-reperfusion injury.Respir Res. 2022 Dec 16;23(1):351. doi: 10.1186/s12931-022-02288-z. Respir Res. 2022. PMID: 36527070 Free PMC article. Review.
-
Primary Graft Dysfunction after Lung Transplantation.Turk J Anaesthesiol Reanim. 2015 Dec;43(6):418-23. doi: 10.5152/TJAR.2015.16443. Epub 2015 Dec 1. Turk J Anaesthesiol Reanim. 2015. PMID: 27366539 Free PMC article. Review.
Cited by
-
Mesenchymal Stromal Cell Therapy in Lung Transplantation.Bioengineering (Basel). 2023 Jun 17;10(6):728. doi: 10.3390/bioengineering10060728. Bioengineering (Basel). 2023. PMID: 37370659 Free PMC article. Review.
-
O-GlcNAcylation attenuates ischemia-reperfusion-induced pulmonary epithelial cell ferroptosis via the Nrf2/G6PDH pathway.BMC Biol. 2025 Feb 4;23(1):32. doi: 10.1186/s12915-025-02126-w. BMC Biol. 2025. PMID: 39901237 Free PMC article.
-
Isolation, culture, and characterization of chicken lung-derived mesenchymal stem cells.Can J Vet Res. 2018 Jul;82(3):225-235. Can J Vet Res. 2018. PMID: 30026648 Free PMC article.
-
The fix is not yet in: recommendation for fixation of lungs within physiological/pathophysiological volume range in preclinical pulmonary structure-function studies.Am J Physiol Lung Cell Mol Physiol. 2024 Aug 1;327(2):L218-L231. doi: 10.1152/ajplung.00341.2023. Epub 2024 May 7. Am J Physiol Lung Cell Mol Physiol. 2024. PMID: 38712433 Free PMC article. Review.
-
Cellular and acellular ex vivo lung perfusion preserve functional lung ultrastructure in a large animal model: a stereological study.Respir Res. 2018 Dec 4;19(1):238. doi: 10.1186/s12931-018-0942-5. Respir Res. 2018. PMID: 30509256 Free PMC article.
References
-
- Andreeva AV, Kutuzov MA, Voyno‐Yasenetskaya TA (2007) Regulation of surfactant secretion in alveolar type II cells. Am J Physiol Lung Cell Mol Physiol 293, L259–L271. - PubMed
-
- Braendgaard H, Gundersen HJ (1986) The impact of recent stereological advances on quantitative studies of the nervous system. J Neurosci Methods 18, 39–78. - PubMed
-
- Brasch F, Johnen G, Winn‐Brasch A, et al. (2004) Surfactant protein B in type II pneumocytes and intra‐alveolar surfactant forms of human lungs. Am J Respir Cell Mol Biol 30, 449–458. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

