Resurgent sodium current promotes action potential firing in the avian auditory brainstem
- PMID: 29193076
- PMCID: PMC5792585
- DOI: 10.1113/JP275083
Resurgent sodium current promotes action potential firing in the avian auditory brainstem
Abstract
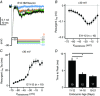
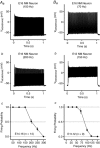
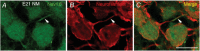
Key points: Auditory brainstem neurons of all vertebrates fire phase-locked action potentials (APs) at high rates with remarkable fidelity, a process controlled by specialized anatomical and biophysical properties. This is especially true in the avian nucleus magnocellularis (NM) - the analogue of the mammalian anteroventral cochlear nucleus. In addition to high voltage-activated potassium (KHVA ) channels, we report, using whole cell physiology and modelling, that resurgent sodium current (INaR ) of sodium channels (NaV ) is equally important and operates synergistically with KHVA channels to enable rapid AP firing in NM. Anatomically, we detected strong NaV 1.6 expression near hearing maturation, which was less distinct during hearing development despite functional evidence of INaR , suggesting that multiple NaV channel subtypes may contribute to INaR . We conclude that INaR plays an important role in regulating rapid AP firing for NM neurons, a property that may be evolutionarily conserved for functions related to similar avian and mammalian hearing.
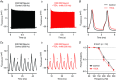
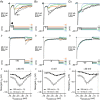
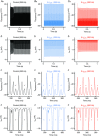
Abstract: Auditory brainstem neurons are functionally primed to fire action potentials (APs) at markedly high rates in order to rapidly encode the acoustic information of sound. This specialization is critical for survival and the comprehension of behaviourally relevant communication functions, including sound localization and distinguishing speech from noise. Here, we investigated underlying ion channel mechanisms essential for high-rate AP firing in neurons of the chicken nucleus magnocellularis (NM) - the avian analogue of bushy cells of the mammalian anteroventral cochlear nucleus. In addition to the established function of high voltage-activated potassium channels, we found that resurgent sodium current (INaR ) plays a role in regulating rapid firing activity of late-developing (embryonic (E) days 19-21) NM neurons. INaR of late-developing NM neurons showed similar properties to mammalian neurons in that its unique mechanism of an 'open channel block state' facilitated the recovery and increased the availability of sodium (NaV ) channels after depolarization. Using a computational model of NM neurons, we demonstrated that removal of INaR reduced high-rate AP firing. We found weak INaR during a prehearing period (E11-12), which transformed to resemble late-developing INaR properties around hearing onset (E14-16). Anatomically, we detected strong NaV 1.6 expression near maturation, which became increasingly less distinct at hearing onset and prehearing periods, suggesting that multiple NaV channel subtypes may contribute to INaR during development. We conclude that INaR plays an important role in regulating rapid AP firing for NM neurons, a property that may be evolutionarily conserved for functions related to similar avian and mammalian hearing.
Keywords: action potential; auditory system; development; neuron; nucleus magnocellularis; potassium channel; sodium channel.
© 2017 University of Oxford. The Journal of Physiology published by John Wiley & Sons Ltd on behalf of The Physiological Society.
Figures





Similar articles
-
Ion channel mechanisms underlying frequency-firing patterns of the avian nucleus magnocellularis: A computational model.Channels (Austin). 2017 Sep 3;11(5):444-458. doi: 10.1080/19336950.2017.1327493. Epub 2017 May 8. Channels (Austin). 2017. PMID: 28481659 Free PMC article.
-
Developmental Profile of Ion Channel Specializations in the Avian Nucleus Magnocellularis.Front Cell Neurosci. 2016 Mar 30;10:80. doi: 10.3389/fncel.2016.00080. eCollection 2016. Front Cell Neurosci. 2016. PMID: 27065805 Free PMC article.
-
Need for Speed and Precision: Structural and Functional Specialization in the Cochlear Nucleus of the Avian Auditory System.J Exp Neurosci. 2018 Dec 12;12:1179069518815628. doi: 10.1177/1179069518815628. eCollection 2018. J Exp Neurosci. 2018. PMID: 30559595 Free PMC article. Review.
-
Diverse Intrinsic Properties Shape Functional Phenotype of Low-Frequency Neurons in the Auditory Brainstem.Front Cell Neurosci. 2018 Jun 26;12:175. doi: 10.3389/fncel.2018.00175. eCollection 2018. Front Cell Neurosci. 2018. PMID: 29997479 Free PMC article.
-
The ion channels and synapses responsible for the physiological diversity of mammalian lower brainstem auditory neurons.Hear Res. 2019 May;376:33-46. doi: 10.1016/j.heares.2018.12.011. Epub 2018 Dec 26. Hear Res. 2019. PMID: 30606624 Review.
Cited by
-
Effects of transient, persistent, and resurgent sodium currents on excitability and spike regularity in vestibular ganglion neurons.Front Neurol. 2024 Nov 18;15:1471118. doi: 10.3389/fneur.2024.1471118. eCollection 2024. Front Neurol. 2024. PMID: 39624672 Free PMC article.
-
Persistent and resurgent Na+ currents in vestibular calyx afferents.J Neurophysiol. 2020 Aug 1;124(2):510-524. doi: 10.1152/jn.00124.2020. Epub 2020 Jul 15. J Neurophysiol. 2020. PMID: 32667253 Free PMC article.
-
Functional Development of Principal Neurons in the Anteroventral Cochlear Nucleus Extends Beyond Hearing Onset.Front Cell Neurosci. 2019 Mar 28;13:119. doi: 10.3389/fncel.2019.00119. eCollection 2019. Front Cell Neurosci. 2019. PMID: 30983974 Free PMC article.
-
Characterization in Effective Stimulation on the Magnitude, Gating, Frequency Dependence, and Hysteresis of INa Exerted by Picaridin (or Icaridin), a Known Insect Repellent.Int J Mol Sci. 2022 Aug 26;23(17):9696. doi: 10.3390/ijms23179696. Int J Mol Sci. 2022. PMID: 36077093 Free PMC article.
-
New Animal Models for Understanding FMRP Functions and FXS Pathology.Cells. 2022 May 12;11(10):1628. doi: 10.3390/cells11101628. Cells. 2022. PMID: 35626665 Free PMC article. Review.
References
-
- Afshari FS, Ptak K, Khaliq ZM, Grieco TM, Slater NT, McCrimmon DR & Raman IM (2004). Resurgent Na currents in four classes of neurons of the cerebellum. J Neurophysiol 92, 2831–2843. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

