Sacubitril/valsartan reduces serum uric acid concentration, an independent predictor of adverse outcomes in PARADIGM-HF
- PMID: 29193563
- PMCID: PMC6607477
- DOI: 10.1002/ejhf.1056
Sacubitril/valsartan reduces serum uric acid concentration, an independent predictor of adverse outcomes in PARADIGM-HF
Abstract
Aims: Elevated serum uric acid concentration (SUA) has been associated with an increased risk of cardiovascular disease, but this may be due to unmeasured confounders. We examined the association between SUA and outcomes as well as the effect of sacubitril/valsartan on SUA in patients with heart failure with reduced ejection fraction (HFrEF) in PARADIGM-HF.
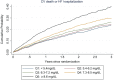
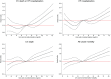
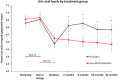
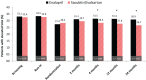
Methods and results: The association between SUA and the primary composite outcome of cardiovascular death or heart failure (HF) hospitalization, its components, and all-cause mortality was examined using Cox regression analyses among 8213 patients using quintiles (Q1-Q5) of SUA adjusted for baseline prognostic variables including estimated glomerular filtration rate (eGFR), diuretic dose, and log N-terminal pro-brain natriuretic peptide. Change in SUA from baseline over 12 months was also evaluated in each treatment group. Patients in Q5 (SUA ≥8.6 mg/dL) compared with Q1 (<5.4 mg/dL) were younger (62.8 vs. 64.2 years), more often male (88.7% vs. 63.1%), had lower systolic blood pressure (119 vs. 123 mmHg), lower eGFR (57.4 vs. 76.6 mL/min/1.73 m2 ), and greater diuretic use. Higher SUA was associated with a higher risk of the primary outcome (adjusted hazard ratios) Q5 vs. Q1 = 1.28 [95% confidence intervals (1.09-1.50), P = 0.003], cardiovascular death [1.44 (1.11-1.77), P = 0.001], HF hospitalization [1.37 (1.11-1.70), P = 0.004], and all-cause mortality [1.36 (1.13-1.64), P = 0.001]. Compared with enalapril, sacubitril/valsartan reduced SUA by 0.24 (0.17-0.32) mg/dL over 12 months (P < 0.0001). Sacubitril/valsartan improved outcomes, irrespective of SUA concentration.
Conclusion: Serum uric acid concentration was an independent predictor of worse outcomes after multivariable adjustment in patients with HFrEF. Compared with enalapril, sacubitril/valsartan reduced SUA and improved outcomes irrespective of SUA.
Keywords: Angiotensin; Heart failure; Mortality; Neprilysin; Uric acid.
© 2017 The Authors. European Journal of Heart Failure published by John Wiley © Sons Ltd on behalf of European Society of Cardiology.
Figures
Comment in
-
Uric acid is important, but there is something that matters even more: to deliver sacubitril/valsartan to eligible heart failure patients.Eur J Heart Fail. 2018 Mar;20(3):523-524. doi: 10.1002/ejhf.1087. Epub 2018 Jan 12. Eur J Heart Fail. 2018. PMID: 29327807 No abstract available.
References
-
- Bergamini C, Cicoira M, Rossi A, Vassanelli C. Oxidative stress and hyperuricaemia: pathophysiology, clinical relevance, and therapeutic implications in chronic heart failure. Eur J Heart Fail 2009;11:444–452. - PubMed
-
- Kanellis J, Kang DH. Uric acid as a mediator of endothelial dysfunction, inflammation, and vascular disease. Semin Nephrol 2005;25:39–42. - PubMed
-
- Anker SD, Doehner W, Rauchhaus M, Sharma R, Francis D, Knosalla C, Davos CH, Cicoira M, Shamim W, Kemp M, Segal R, Osterziel KJ, Leyva F, Hetzer R, Ponikowski P, Coats AJ. Uric acid and survival in chronic heart failure: validation and application in metabolic, functional, and hemodynamic staging. Circulation 2003;107:1991–1997. - PubMed
-
- Hare JM, Johnson RJ. Uric acid predicts clinical outcomes in heart failure: insights regarding the role of xanthine oxidase and uric acid in disease pathophysiology. Circulation 2003;107:1951–1953. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

