A direct role for hepatitis B virus X protein in inducing mitochondrial membrane permeabilization
- PMID: 29193612
- PMCID: PMC7167162
- DOI: 10.1111/jvh.12831
A direct role for hepatitis B virus X protein in inducing mitochondrial membrane permeabilization
Abstract
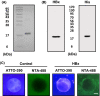
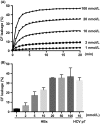
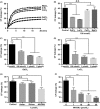
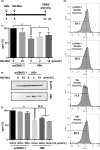
Hepatitis B virus X protein (HBx) acts as a multifunctional protein that regulates intracellular signalling pathways during HBV infection. It has mainly been studied in terms of its interaction with cellular proteins. Here, we show that HBx induces membrane permeabilization independently of the mitochondrial permeability transition pore complex. We generated mitochondrial outer membrane-mimic liposomes to observe the direct effects of HBx on membranes. We found that HBx induced membrane permeabilization, and the region comprising the transmembrane domain and the mitochondrial-targeting sequence was sufficient for this process. Membrane permeabilization was inhibited by nonselective channel blockers or by N-(n-nonyl)deoxynojirimycin (NN-DNJ), a viroporin inhibitor. Moreover, NN-DNJ inhibited HBx-induced mitochondrial depolarization in Huh-7 cells. Based on the results of this study, we can postulate that the HBx protein itself is sufficient to induce mitochondrial membrane permeabilization. Our finding provides important information for a strategy of HBx targeting during HBV treatment.
Keywords: hepatitis B virus X protein; membrane permeabilization; viroporin.
© 2017 John Wiley & Sons Ltd.
Figures

Similar articles
-
Hepatitis B virus X protein induces size-selective membrane permeabilization through interaction with cardiolipin.Biochim Biophys Acta Biomembr. 2019 Apr 1;1861(4):729-737. doi: 10.1016/j.bbamem.2019.01.006. Epub 2019 Jan 16. Biochim Biophys Acta Biomembr. 2019. PMID: 30658058
-
Hepatitis B virus HBx protein localizes to mitochondria in primary rat hepatocytes and modulates mitochondrial membrane potential.J Virol. 2008 Jul;82(14):6798-811. doi: 10.1128/JVI.00154-08. Epub 2008 Apr 30. J Virol. 2008. PMID: 18448529 Free PMC article.
-
Hepatitis B virus X protein modulates apoptosis in primary rat hepatocytes by regulating both NF-kappaB and the mitochondrial permeability transition pore.J Virol. 2009 May;83(10):4718-31. doi: 10.1128/JVI.02590-08. Epub 2009 Mar 11. J Virol. 2009. PMID: 19279112 Free PMC article.
-
The molecular and pathophysiological implications of hepatitis B X antigen in chronic hepatitis B virus infection.Rev Med Virol. 2011 Sep;21(5):315-29. doi: 10.1002/rmv.699. Epub 2011 Jul 14. Rev Med Virol. 2011. PMID: 21755567 Review.
-
Identifying and Characterizing Interplay between Hepatitis B Virus X Protein and Smc5/6.Viruses. 2017 Apr 3;9(4):69. doi: 10.3390/v9040069. Viruses. 2017. PMID: 28368357 Free PMC article. Review.
Cited by
-
Mitochondria in the biology, pathogenesis, and treatment of hepatitis virus infections.Rev Med Virol. 2019 Sep;29(5):e2075. doi: 10.1002/rmv.2075. Epub 2019 Jul 19. Rev Med Virol. 2019. PMID: 31322806 Free PMC article. Review.
-
The Malignant Transformation of Viral Hepatitis to Hepatocellular Carcinoma: Mechanisms and Interventions.MedComm (2020). 2025 Mar 8;6(3):e70121. doi: 10.1002/mco2.70121. eCollection 2025 Mar. MedComm (2020). 2025. PMID: 40060195 Free PMC article. Review.
-
Romo1-Derived Antimicrobial Peptide Is a New Antimicrobial Agent against Multidrug-Resistant Bacteria in a Murine Model of Sepsis.mBio. 2020 Apr 14;11(2):e03258-19. doi: 10.1128/mBio.03258-19. mBio. 2020. PMID: 32291307 Free PMC article.
-
The 6-kilodalton peptide 1 of the family Potyviridae: small in size but powerful in function.Front Microbiol. 2025 Jun 5;16:1605199. doi: 10.3389/fmicb.2025.1605199. eCollection 2025. Front Microbiol. 2025. PMID: 40539110 Free PMC article. Review.
-
HBxAg promotes HBV replication and EGFR activation in human placental trophoblasts.Exp Ther Med. 2021 Nov;22(5):1211. doi: 10.3892/etm.2021.10645. Epub 2021 Aug 24. Exp Ther Med. 2021. PMID: 34584556 Free PMC article.
References
-
- Lau JY, Wright TL. Molecular virology and pathogenesis of hepatitis B. Lancet. 1993;342:1311‐1340. - PubMed
-
- Lucifora J, Protzer U. Hepatitis B virus X protein: a key regulator of the virus life cycle. Rijeka, Croatia: INTECH Open Access Publisher; 2012.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

