Negative regulators of platelet activation and adhesion
- PMID: 29193689
- PMCID: PMC5809258
- DOI: 10.1111/jth.13910
Negative regulators of platelet activation and adhesion
Abstract
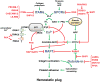
Platelets are small anucleated cells that constantly patrol the cardiovascular system to preserve its integrity and prevent excessive blood loss where the vessel lining is breached. Their key challenge is to form a hemostatic plug under conditions of high shear forces. To do so, platelets have evolved a molecular machinery that enables them to sense trace amounts of signals at the site of damage and to rapidly shift from a non-adhesive to a pro-adhesive state. However, this highly efficient molecular machinery can also lead to unintended platelet activation and cause clinical complications such as thrombocytopenia and thrombosis. Thus, several checkpoints are in place to tightly control platelet activation and adhesiveness in space and time. In this review, we will discuss select negative regulators of platelet activation, which are critical to maintain patrolling platelets in a quiescent, non-adhesive state and/or to limit platelet adhesion to sites of injury.
Keywords: hemostasis; negative regulators; platelet adhesion; platelet reactivity; thrombosis.
© 2017 International Society on Thrombosis and Haemostasis.
Conflict of interest statement
W. Bergmeier reports grants from Merck outside the submitted work.
Authors report no conflicts of interests.
Figures

References
-
- Aarts PA, van den Broek SA, Prins GW, Kuiken GD, Sixma JJ, Heethaar RM. Blood platelets are concentrated near the wall and red blood cells, in the center in flowing blood. Arteriosclerosis. 1988;8:819–24. - PubMed
-
- Schmaier AA, Zou Z, Kazlauskas A, Emert-Sedlak L, Fong KP, Neeves KB, Maloney SF, Diamond SL, Kunapuli SP, Ware J, Brass LF, Smithgall TE, Saksela K, Kahn ML. Molecular priming of Lyn by GPVI enables an immune receptor to adopt a hemostatic role. Proc Natl Acad Sci USA. 2009;106:21167–72. - PMC - PubMed
-
- Heemskerk JWM, Harper MT, Cosemans JMEM, Poole AW. Unravelling the different functions of protein kinase C isoforms in platelets. FEBS Lett. 2011;585:1711–6. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

