Reverse Translation in Advancing Pharmacotherapy in Pediatric Rheumatology: A Logical Approach in Rare Diseases with Limited Resources
- PMID: 29193724
- PMCID: PMC5866971
- DOI: 10.1111/cts.12513
Reverse Translation in Advancing Pharmacotherapy in Pediatric Rheumatology: A Logical Approach in Rare Diseases with Limited Resources
Figures

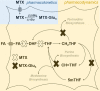
References
-
- Russo, R.A. & Katsicas, M.M. Clinical remission in patients with systemic juvenile idiopathic arthritis treated with anti‐tumor necrosis factor agents. J. Rheumatol. 36, 1078–1082 (2009). - PubMed
-
- de Benedetti, F. et al Correlation of serum interleukin‐6 levels with joint involvement and thrombocytosis in systemic juvenile rheumatoid arthritis. Arthritis Rheum. 34, 1158–1163 (1991). - PubMed
-
- Becker, M.L. et al The effect of genotype on methotrexate polyglutamate variability in juvenile idiopathic arthritis and association with drug response. Arthritis Rheum. 63, 276–285 (2011). - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

