Overexpression of the class I homeodomain transcription factor TaHDZipI-5 increases drought and frost tolerance in transgenic wheat
- PMID: 29193733
- PMCID: PMC5978581
- DOI: 10.1111/pbi.12865
Overexpression of the class I homeodomain transcription factor TaHDZipI-5 increases drought and frost tolerance in transgenic wheat
Abstract
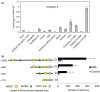
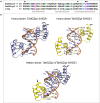
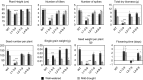
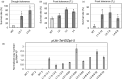
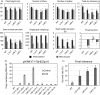
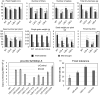
Characterization of the function of stress-related genes helps to understand the mechanisms of plant responses to environmental conditions. The findings of this work defined the role of the wheat TaHDZipI-5 gene, encoding a stress-responsive homeodomain-leucine zipper class I (HD-Zip I) transcription factor, during the development of plant tolerance to frost and drought. Strong induction of TaHDZipI-5 expression by low temperatures, and the elevated TaHDZipI-5 levels of expression in flowers and early developing grains in the absence of stress, suggests that TaHDZipI-5 is involved in the regulation of frost tolerance at flowering. The TaHDZipI-5 protein behaved as an activator in a yeast transactivation assay, and the TaHDZipI-5 activation domain was localized to its C-terminus. The TaHDZipI-5 protein homo- and hetero-dimerizes with related TaHDZipI-3, and differences between DNA interactions in both dimers were specified at 3D molecular levels. The constitutive overexpression of TaHDZipI-5 in bread wheat significantly enhanced frost and drought tolerance of transgenic wheat lines with the appearance of undesired phenotypic features, which included a reduced plant size and biomass, delayed flowering and a grain yield decrease. An attempt to improve the phenotype of transgenic wheat by the application of stress-inducible promoters with contrasting properties did not lead to the elimination of undesired phenotype, apparently due to strict spatial requirements for TaHDZipI-5 overexpression.
Keywords: 3D protein modelling; abiotic stress; activation domain; phenotypic features; protein homo- and hetero-dimerization; stress-inducible promoters.
© 2017 The Authors. Plant Biotechnology Journal published by Society for Experimental Biology and The Association of Applied Biologists and John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Agalou, A. , Purwantomo, S. , Overnas, E. , Johannesson, H. , Zhu, X. , Estiati, A. , Kam, R.J.D. et al. (2008) A genome‐wide survey of HD‐Zip genes in rice and analysis of drought‐responsive family members. Plant Mol. Biol. 66, 87–103. - PubMed
-
- Agarwal, P.K. , Gupta, K. , Lopato, S. and Agarwal, P. (2017) Dehydration responsive element binding transcription factors and their applications for the engineering of stress tolerance. J. Exp. Bot. 68, 2135–2148. - PubMed
-
- Aharoni, A. , Dixit, S. , Jetter, R. , Thoenes, E. , Arkel, G.V. and Pereira, A. (2004) The SHINE clade of AP2 domain transcription factors activates wax biosynthesis, alters cuticle properties, and confers drought tolerance when overexpressed in Arabidopsis . Plant Cell, 16, 2463–2480. - PMC - PubMed
-
- Ariel, F.D. , Manavella, P.A. , Dezar, C.A. and Chan, R.L. (2007) The true story of the HD‐Zip family. Trends Plant Sci. 12, 419–426. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources

