Shotgun label-free proteomic analysis for identification of proteins in HaCaT human skin keratinocytes regulated by the administration of collagen from soft-shelled turtle
- PMID: 29193735
- PMCID: PMC6175320
- DOI: 10.1002/jbm.b.34034
Shotgun label-free proteomic analysis for identification of proteins in HaCaT human skin keratinocytes regulated by the administration of collagen from soft-shelled turtle
Abstract
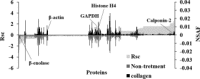
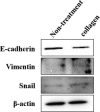
Soft-shelled turtles (Pelodiscus sinensis) are widely distributed in some Asian countries, and we previously reported that soft-shelled turtle tissue could be a useful material for collagen. In the present study, we performed shotgun liquid chromatography (LC)/mass spectrometry (MS)-based global proteomic analysis of collagen-administered human keratinocytes to examine the functional effects of collagen from soft-shelled turtle on human skin. Using a semiquantitative method based on spectral counting, we were able to successfully identify 187 proteins with expression levels that were changed more than twofold by the administration of collagen from soft-shelled turtle. Based on Gene Ontology analysis, the functions of these proteins closely correlated with cell-cell adhesion. In addition, epithelial-mesenchymal transition was induced by the administration of collagen from soft-shelled turtle through the down-regulation of E-cadherin expression. Moreover, collagen-administered keratinocytes significantly facilitated wound healing compared with nontreated cells in an in vitro scratch wound healing assay. These findings suggest that collagen from soft-shelled turtle provides significant benefits for skin wound healing and may be a useful material for pharmaceuticals and medical care products. © 2017 The Authors Journal of Biomedical Materials Research Part B: Applied Biomaterials Published by Wiley Periodicals, Inc. J Biomed Mater Res Part B: Appl Biomater, 106B: 2403-2413, 2018.
Keywords: E-cadherin; collagen; epithelial-mesenchymal transition; soft-shelled turtle; wound healing.
© 2017 The Authors Journal of Biomedical Materials Research Part B: Applied Biomaterials Published by Wiley Periodicals, Inc.
Figures


Similar articles
-
Collagen peptides from soft‑shelled turtle induce calpain‑1 expression and regulate inflammatory cytokine expression in HaCaT human skin keratinocytes.Int J Mol Med. 2018 Aug;42(2):1168-1180. doi: 10.3892/ijmm.2018.3659. Epub 2018 May 8. Int J Mol Med. 2018. PMID: 29750291
-
Comparative study of collagen distribution in the dermis of the embryonic carapace of soft- and hard-shelled cryptodiran turtles.J Morphol. 2021 Apr;282(4):543-552. doi: 10.1002/jmor.21327. Epub 2021 Feb 8. J Morphol. 2021. PMID: 33491791
-
Identification and application of species-specific peptide biomarkers from soft-shelled turtles (Pelodiscus sinensis) using post-translational modification detection-based peptidomics analysis.Food Chem. 2023 Sep 1;419:135983. doi: 10.1016/j.foodchem.2023.135983. Epub 2023 Mar 21. Food Chem. 2023. PMID: 37011573
-
Optimization of Method to Extract Collagen from "Emperor" Tissue of Soft-shelled Turtles.J Oleo Sci. 2016;65(2):169-75. doi: 10.5650/jos.ess15220. Epub 2016 Jan 15. J Oleo Sci. 2016. PMID: 26782306
-
Immunization with Bovine Serum Albumin (BSA) in Oil-Adjuvant Elicits IgM Antibody Response in Chinese Soft-Shelled Turtle (Pelodiscus Sinensis).Vaccines (Basel). 2020 May 29;8(2):257. doi: 10.3390/vaccines8020257. Vaccines (Basel). 2020. PMID: 32485925 Free PMC article.
References
-
- Gelse K, Poschl E, Aigner T. Collagens–structure, function, and biosynthesis. Adv Drug Deliv Rev 2003;55(12):1531–1546. - PubMed
-
- Myllyharju J, Kivirikko KI. Collagens, modifying enzymes and their mutations in humans, flies and worms. Trends Genet 2004;20(1):33–43. - PubMed
-
- Adachi E, Hayashi T. Anchoring of epithelia to underlying connective tissue: Evidence of frayed ends of collagen fibrils directly merging with meshwork of lamina densa. J Electron Microsc (Tokyo) 1994;43(5):264–271. - PubMed
-
- Park KH, Bae YH. Phenotype of hepatocyte spheroids in Arg‐GLY‐Asp (RGD) containing a thermo‐reversible extracellular matrix. Biosci Biotechnol Biochem 2002;66(7):1473–1478. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

