Urine RNA Processing in a Clinical Setting: Comparison of 3 Protocols
- PMID: 29194081
- PMCID: PMC5975100
- DOI: 10.1097/SPV.0000000000000525
Urine RNA Processing in a Clinical Setting: Comparison of 3 Protocols
Abstract
Objective: The objective of this study was to compare quantitative and qualitative RNA extraction results from clinical voided urine samples between 3 commercially available extraction protocols.
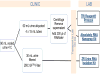
Methods: For phase 1, fresh voided urine samples from 10 female subjects were collected and processed in clinic and transported to the laboratory with cold packs. RNA was purified with 1 of 3 RNA extraction protocols: (1) TRI Reagent Protocol; (2) Absolutely RNA Nanoprep Kit; and (3) ZR Urine RNA Isolation Kit. Real-time polymerase chain reactions (RT-PCR) were performed. As the ZR Urine RNA Isolation Kit provided the highest quality RNA in phase 1, for phase 2, RNA was extracted from 9 additional voided urine specimens using this kit to perform additional qualitative analyses.
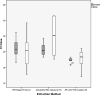
Results: Median RNA yield was significantly higher with the TRI Reagent Protocol as compared with the other protocols (P = 0.007). However, there was a significantly lower median threshold cycle value from polymerase chain reaction (indicating improved downstream application performance) with the ZR Urine RNA Isolation Kit as compared with the other methods (P = 0.005). In phase 2, the median RNA integrity number of urine RNA was 2.5 (range, 1.6-5.9).
Conclusions: Although other methods may provide a higher quantity of RNA, when using clinical urine samples, the ZR Urine RNA Isolation Kit provided the highest quality of extracted RNA. This kit is especially attractive for the clinical setting because it does not require an initial centrifugation step. The urine RNA obtained with this kit may be useful for polymerase chain reaction but is not likely to be of high enough integrity for RNA sequencing.
Conflict of interest statement
Figures


References
-
- Erickson DR, S S, Dixon JK, Clark CJ, Hersh MA. Differentiation Associated Changes in Gene Expression Profiles of Interstitial Cystitis and Control Urothelial cells. J Urol. 2008;180:2681–2687. - PubMed
-
- Colaco M, Koslov DS, Keys T, et al. Correlation of Gene Expression with Bladder Capacity in Interstitial Cystitis/Bladder Pain Syndrome. J Urol. 2014;192:1123–1129. - PubMed
-
- Blalock EM, Korrect GS, Stromberg AJ, Erickson DR. Gene Expression Analysis of Urine Sediment: Evaluation for Potential Noninvasive Markers of Interstitial Cystitis/Bladder Pain Syndrome. J Urol. 2012;187:725–732. - PubMed
-
- Medeiros M, Sharma VK, Ding R, et al. Optimization of RNA yield, purity and mRNA copy number by treatment of urine cell pellets with RNAlater. J Immunol Methods. 2003 Aug;279(1–2):135–142. - PubMed
-
- Crossland RE, Norden J, Bibby LA, Davis J, Dickinson AM. Evaluation of optimal extracellular vesicle small RNA isolation and qRT-PCR normalisation for serum and urine. J Immunol Methods. 2016 Feb;429:39–49. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

