Trends in antiretroviral therapy prescription, durability and modification: new drugs, more changes, but less failure
- PMID: 29194118
- PMCID: PMC6604808
- DOI: 10.1097/QAD.0000000000001708
Trends in antiretroviral therapy prescription, durability and modification: new drugs, more changes, but less failure
Abstract
Objective: To evaluate the real world durability of contemporary ART for treatment-naïve people living with HIV (PLWH).
Design: A retrospective follow-up study in a multisite cohort.
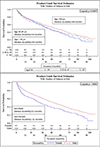
Methods: This study of the CNICS (CFAR Network of Integrated Clinical Systems) cohort integrates data from eight Center for AIDS Research (CFARs). PLWH initiating ART between 2007 and 2014 were included. Durability was defined as time from the initiation until discontinuation/modification using Kaplan-Meier survival curves. Cox Proportional Hazards measured associations with various sociodemographic and clinical characteristics.
Results: Among 5373 PLWH, the initial regimen was modified in 2285 (43%) patients. Efavirenz/emtricitabine/tenofovir (n = 2173, 40%) was the most commonly prescribed initial ART regimen; elvitegravir/cobicistat/emtricitabine/tenofovir became more common after 2012. Median durability for all regimens was 48.6 months. There were statistically significant differences in median durability for NNRTI, InSTI, and protease inhibitor-based regimens, which lasted 61, 44, and 32 months, respectively. Female sex (aHR = 1.4; 95% CI 1.2-1.6), intravenous drug use (aHR = 1.6; 95% CI 1.3-1.9), and CD4 cell count less than 200 cells/μl (aHR = 1.2; 95% CI 1.1-1.3) were significantly associated with regimen modification. Compared with InSTI, those receiving an InSTI/protease inhibitor (aHR = 2.7; 95% CI 2.0-3.7) or protease inhibitor-based ART (aHR = 1.9; 95% CI 1.6-2.2) were significantly more likely to be modified; but those receiving NNRTI (aHR = 1.1; 95% CI 0.9-1.3) were not.
Conclusion: In treatment-naive PLWH, NNRTI and InSTI-based ART were most durable, relative to protease inhibitor and InSTI/protease inhibitor-based ART, and were least likely to be modified/discontinued. A greater understanding of reasons for regimen modification/discontinuation is needed to analyze contemporary regimen durability.
Figures


References
-
- DHHS. Guidelines for the Use of Antiretroviral Agents in HIV-1-Infected Adults and Adolescents. [cited 2016 August 10]; Available from: http://aidsinfo.nih.gov/guidelines/html/1/adult-and-adolescent-arv-guide....
-
- Clotet B, et al., Once-daily dolutegravir versus darunavir plus ritonavir in antiretroviral-naive adults with HIV-1 infection (FLAMINGO): 48 week results from the randomised open-label phase 3b study. Lancet, 2014. 383(9936): p. 2222–31. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

