Olfactory coding in the turbulent realm
- PMID: 29194457
- PMCID: PMC5736211
- DOI: 10.1371/journal.pcbi.1005870
Olfactory coding in the turbulent realm
Abstract
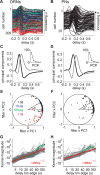
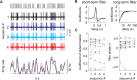
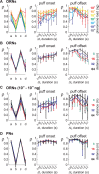
Long-distance olfactory search behaviors depend on odor detection dynamics. Due to turbulence, olfactory signals travel as bursts of variable concentration and spacing and are characterized by long-tail distributions of odor/no-odor events, challenging the computing capacities of olfactory systems. How animals encode complex olfactory scenes to track the plume far from the source remains unclear. Here we focus on the coding of the plume temporal dynamics in moths. We compare responses of olfactory receptor neurons (ORNs) and antennal lobe projection neurons (PNs) to sequences of pheromone stimuli either with white-noise patterns or with realistic turbulent temporal structures simulating a large range of distances (8 to 64 m) from the odor source. For the first time, we analyze what information is extracted by the olfactory system at large distances from the source. Neuronal responses are analyzed using linear-nonlinear models fitted with white-noise stimuli and used for predicting responses to turbulent stimuli. We found that neuronal firing rate is less correlated with the dynamic odor time course when distance to the source increases because of improper coding during long odor and no-odor events that characterize large distances. Rapid adaptation during long puffs does not preclude however the detection of puff transitions in PNs. Individual PNs but not individual ORNs encode the onset and offset of odor puffs for any temporal structure of stimuli. A higher spontaneous firing rate coupled to an inhibition phase at the end of PN responses contributes to this coding property. This allows PNs to decode the temporal structure of the odor plume at any distance to the source, an essential piece of information moths can use in their tracking behavior.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures






References
-
- Nagel KI, Wilson RI. Biophysical mechanisms underlying olfactory receptor neuron dynamics. Nat Neurosci. 2011;14(2):208–16. doi: 10.1038/nn.2725 - DOI - PMC - PubMed
-
- Riffell JA, Shlizerman E, Sanders E, Abrell L, Medina B, Hinterwirth AJ, et al. Flower discrimination by pollinators in a dynamic chemical environment. Science. 2014;344(6191):1515–8. doi: 10.1126/science.1251041 - DOI - PubMed
-
- Vickers NJ, Christensen TA, Baker TC, Hildebrand JG. Odour-plume dynamics influence the brain's olfactory code. Nature. 2001;410(6827):466–70. doi: 10.1038/35068559 - DOI - PubMed
-
- Gorur-Shandilya S, Demir M, Long J, Clark DA, Emonet T. Olfactory receptor neurons use gain control and complementary kinetics to encode intermittent odorant stimuli. Elife. 2017;6:e27670 doi: 10.7554/eLife.27670 - DOI - PMC - PubMed
-
- Falkovich G, Gawedzki K, Vergassola M. Particles and fields in fluid turbulence. Rev Mod Phys. 2001;73(4):913–75.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

