Translational Development of Microbiome-Based Therapeutics: Kinetics of E. coli Nissle and Engineered Strains in Humans and Nonhuman Primates
- PMID: 29194983
- PMCID: PMC5866967
- DOI: 10.1111/cts.12528
Translational Development of Microbiome-Based Therapeutics: Kinetics of E. coli Nissle and Engineered Strains in Humans and Nonhuman Primates
Abstract
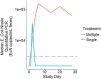
Understanding the pharmacology of microbiome-based therapeutics is required to support the development of new medicines. Strains of E. coli Nissle (EcN) were genetically modified and administered to cynomolgus monkeys at doses of 1 × 109 and 1 × 1012 colony-forming units (CFU)/day for 28 days. A clinical study to evaluate the exposure and clearance of EcN in healthy volunteers was also performed. Healthy subjects received oral doses of EcN, 2.5 to 25 × 109 CFU 3 times daily for 28 days or a single day. In cynomolgus monkeys, replicating strains yielded higher fecal concentrations than nonreplicating strains and persisted for longer following cessation of dosing. In the clinical study, all subjects cleared EcN following cessation of dosing with median clearance of 1 week. Quantitative methodology can be applied to microbiome-based therapeutics, and similar kinetics and clearance were observed for EcN in cynomolgus monkeys and humans.
© 2017 The Authors. Clinical and Translational Science published by Wiley Periodicals, Inc. on behalf of American Society for Clinical Pharmacology and Therapeutics.
Figures



References
-
- Joeres‐Nguyen‐Xuan, T.H. , Boehm, S.K. , Joeres, L. , Schulze, J. & Kruis, W. Survival of the probiotic Escherichia coli Nissle 1917 (EcN) in the gastrointestinal tract given in combination with oral mesalamine to healthy volunteers. Inflamm. Bowel Dis. 16, 256–262 (2010). - PubMed
-
- Lata, J. et al Effect of administration of Escherichia coli Nissle (Mutaflor) on intestinal colonization, endotoxemia, liver function and minimal hepatic encephalopathy in patients with liver cirrhosis. Vnitr. Lek. 52, 215–219 (2006). - PubMed
-
- Wassenaar, T.M. , Beimfohr, C. , Geske, T. & Zimmermann, K. Voluntarily exposure to a single, high dose of probiotic Escherichia coli results in prolonged colonisation. Benef. Microbes 5, 367–375 (2014). - PubMed
-
- Cukrowska, B. , Lodlnová‐Zádnlková, R. , Enders, C. , Sonnenborn, U. , Schulze, J. & Tlaskalová‐Hogenová, H. Specific proliferative and antibody responses of premature infants to intestinal colonization with nonpathogenic probiotic E. coli strain Nissle 1917. Scand. J. Immunol. 55, 204–209 (2002). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

