Epsin-Dependent Ligand Endocytosis Activates Notch by Force
- PMID: 29195077
- PMCID: PMC6219616
- DOI: 10.1016/j.cell.2017.10.048
Epsin-Dependent Ligand Endocytosis Activates Notch by Force
Abstract
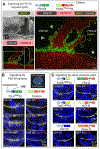
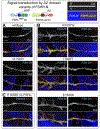
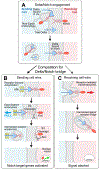
DSL ligands activate Notch by inducing proteolytic cleavage of the receptor ectodomain, an event that requires ligand to be endocytosed in signal-sending cells by the adaptor protein Epsin. Two classes of explanation for this unusual requirement are (1) recycling models, in which the ligand must be endocytosed to be modified or repositioned before it binds Notch and (2) pulling models, in which the ligand must be endocytosed after it binds Notch to exert force that exposes an otherwise buried site for cleavage. We demonstrate in vivo that ligands that cannot enter the Epsin pathway nevertheless bind Notch but fail to activate the receptor because they cannot exert sufficient force. This argues against recycling models and in favor of pulling models. Our results also suggest that once ligand binds receptor, activation depends on a competition between Epsin-mediated ligand endocytosis, which induces cleavage, and transendocytosis of the ligand by the receptor, which aborts the incipient signal.
Keywords: DSL/Notch signaling; Notch; clathrin; delta; endocytosis; epsin; force; von Willebrand factor.
Copyright © 2017 Elsevier Inc. All rights reserved.
Figures




References
-
- Bang AG, Bailey AM, and Posakony JW (1995). Hairless promotes stable commitment to the sensory organ precursor cell fate by negatively regulating the activity of the Notch signaling pathway. Dev. Biol. 172, 479–494. - PubMed
-
- Blair SS (1997). Limb development: marginal fringe benefits. Curr. Biol. 7, R686–R690. - PubMed
-
- Cagan RL, Krämer H, Hart AC, and Zipursky SL (1992). The bride of sevenless and sevenless interaction: internalization of a transmembrane ligand. Cell 69, 393–399. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

