Insulin and branched-chain amino acid depletion during mouse preimplantation embryo culture programmes body weight gain and raised blood pressure during early postnatal life
- PMID: 29196239
- PMCID: PMC5764225
- DOI: 10.1016/j.bbadis.2017.11.020
Insulin and branched-chain amino acid depletion during mouse preimplantation embryo culture programmes body weight gain and raised blood pressure during early postnatal life
Abstract
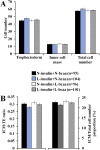
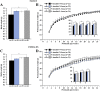
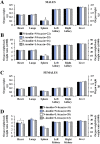
Mouse maternal low protein diet exclusively during preimplantation development (Emb-LPD) is sufficient to programme altered growth and cardiovascular dysfunction in offspring. Here, we use an in vitro model comprising preimplantation culture in medium depleted in insulin and branched-chain amino acids (BCAA), two proposed embryo programming inductive factors from Emb-LPD studies, to examine the consequences for blastocyst organisation and, after embryo transfer (ET), postnatal disease origin. Two-cell embryos were cultured to blastocyst stage in defined KSOM medium supplemented with four combinations of insulin and BCAA concentrations. Control medium contained serum insulin and uterine luminal fluid amino acid concentrations (including BCAA) found in control mothers from the maternal diet model (N-insulin+N-bcaa). Experimental medium (three groups) contained 50% reduction in insulin and/or BCAA (L-insulin+N-bcaa, N-insulin+L-bcaa, and L-insulin+N-bcaa). Lineage-specific cell numbers of resultant blastocysts were not affected by treatment. Following ET, a combined depletion of insulin and BCAA during embryo culture induced a non sex-specific increase in birth weight and weight gain during early postnatal life. Furthermore, male offspring displayed relative hypertension and female offspring reduced heart/body weight, both characteristics of Emb-LPD offspring. Combined depletion of metabolites also resulted in a strong positive correlation between body weight and glucose metabolism that was absent in the control group. Our results support the notion that composition of preimplantation culture medium can programme development and associate with disease origin affecting postnatal growth and cardiovascular phenotypes and implicate two important nutritional mediators in the inductive mechanism. Our data also have implications for human assisted reproductive treatment (ART) practice.
Keywords: Birth weight; Blastocyst; Branched-chain amino acids; DOHaD (developmental origins of health and disease); Insulin; Systolic blood pressure.
Copyright © 2017 The Authors. Published by Elsevier B.V. All rights reserved.
Figures



References
-
- Velazquez M.A., Fleming T.P. Maternal diet, oocyte nutrition and metabolism, and offspring health. In: Coticchio G., Albertini F.D., De Santis L., editors. Oogenesis. Springer London; London: 2013. pp. 329–351.
-
- Barker D.J. The origins of the developmental origins theory. J. Intern. Med. 2007;261:412–417. - PubMed
-
- Langley-Evans S.C., McMullen S. Developmental origins of adult disease. Med. Princ. Pract. 2010;19:87–98. - PubMed
-
- Fleming T.P., Watkins A.J., Sun C., Velazquez M.A., Smyth N.R., Eckert J.J. Do little embryos make big decisions? How maternal dietary protein restriction can permanently change an embryo's potential, affecting adult health. Reprod. Fertil. Dev. 2015;27:684–692. - PubMed
-
- Watkins A.J., Ursell E., Panton R., Papenbrock T., Hollis L., Cunningham C., Wilkins A., Perry V.H., Sheth B., Kwong W.Y., Eckert J.J., Wild A.E., Hanson M.A., Osmond C., Fleming T.P. Adaptive responses by mouse early embryos to maternal diet protect fetal growth but predispose to adult onset disease. Biol. Reprod. 2008;78:299–306. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- MC_UU_12011/3/MRC_/Medical Research Council/United Kingdom
- MC_UU_12011/4/MRC_/Medical Research Council/United Kingdom
- MC_UP_A620_1016/MRC_/Medical Research Council/United Kingdom
- BB/F007450/1/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- MC_U147574242/MRC_/Medical Research Council/United Kingdom
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

