Local Inhibition of PERK Enhances Memory and Reverses Age-Related Deterioration of Cognitive and Neuronal Properties
- PMID: 29196323
- PMCID: PMC6596193
- DOI: 10.1523/JNEUROSCI.0628-17.2017
Local Inhibition of PERK Enhances Memory and Reverses Age-Related Deterioration of Cognitive and Neuronal Properties
Abstract
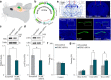
Protein kinase R (PKR)-like endoplasmic reticulum kinase (PERK) is one of four known kinases that respond to cellular stress by deactivating the eukaryotic initiation factor 2 α (eIF2α) or other signal transduction cascades. Recently, both eIF2α and its kinases were found to play a role in normal and pathological brain function. Here, we show that reduction of either the amount or the activity of PERK, specifically in the CA1 region of the hippocampus in young adult male mice, enhances neuronal excitability and improves cognitive function. In addition, this manipulation rescues the age-dependent cellular phenotype of reduced excitability and memory decline. Specifically, the reduction of PERK expression in the CA1 region of the hippocampus of middle-aged male mice using a viral vector rejuvenates hippocampal function and improves hippocampal-dependent learning. These results delineate a mechanism for behavior and neuronal aging and position PERK as a promising therapeutic target for age-dependent brain malfunction.SIGNIFICANCE STATEMENT We found that local reduced protein kinase R (PKR)-like endoplasmic reticulum kinase (PERK) expression or activity in the hippocampus enhances neuronal excitability and cognitive function in young normal mice, that old CA1 pyramidal cells have reduced excitability and increased PERK expression that can be rescued by reducing PERK expression in the hippocampus, and that reducing PERK expression in the hippocampus of middle-aged mice enhances hippocampal-dependent learning and memory and restores it to normal performance levels of young mice. These findings uncover an entirely new biological link among PERK, neuronal intrinsic properties, aging, and cognitive function. Moreover, our findings propose a new way to fight mild cognitive impairment and aging-related cognitive deterioration.
Keywords: PERK; aging; intrinsic properties; memory enhancement; translation regulation.
Copyright © 2018 the authors 0270-6474/18/380648-11$15.00/0.
Figures




References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous
